- Confined spaces can contain hazardous gases produced through various causes or migrating from the surrounding environment.
- The three main confined spaces risks include oxygen deficiency, carbon monoxide, and hydrogen sulfide.
- The Occupational Safety and Health Administration (OSHA) requires employees entering permit-required confined spaces to test the air using calibrated gas detection instruments.
Workers in confined spaces face numerous dangers. Hazardous gases are a significant concern, as they are the primary cause of deaths in confined spaces. Hazardous gases that accumulate can also pose safety or health risks. Such confined spaces can be found in many industries, and the types of harmful gases can vary. Therefore, it is essential to understand what these hazardous gases are and how they are formed to mitigate the threat they pose to workers entering confined spaces.
Why are Confined Spaces at More Risk?
Confined spaces are areas not intended for continuous employee occupancy and have limited entry and exit points. Examples of confined spaces are underground structures, storage bins and tanks, tunnels, mines, shafts, vessels, silos, pits, maintenance holes, pipelines, and equipment housing.
Confined spaces can be hazardous because they are used to store materials such as grains, chemicals, or petroleum products that can produce fumes and displace oxygen, creating oxygen depletion. Moreover, being in closed and confined spaces restricts airflow and can accumulate other toxic gases, besides limiting the fresh supply of oxygen. People entering confined areas are in a hazardous atmosphere, as movement and escape are restricted by the structure’s design.
The confined space gases impair workers’ ability to escape, and people can suffer injury, acute illness, or even death. The fatality rate in confined spaces is 0.05 to 0.08 per 100,000 workers, primarily caused by gas pollution and physical hazards. Around 17% of deaths are of would-be rescuers, whose deaths are almost entirely due to toxic gases.
The danger from the gases is due to the following reasons:
- The gases in confined spaces are toxic.
- Flammable gases present in over 10% of the lower flammable limit (LFL) are a safety risk that can cause fires and explosions.
- Combustible dust at its LFL can cause fires and also obscure vision.
- The air’s oxygen concentration is below 19.5% or above 23.5%.
Confined spaces with identified gas hazards are referred to as “Permit-Required Confined Spaces.” In the USA, the Occupational Safety and Health Administration (OSHA) standard 29 CFR 1910.146(c) requires employees entering permit-required confined spaces to test the air with calibrated instruments that show actionable measurements of oxygen content, flammable vapors and gases, and toxic contaminants.
Sources of Hazardous Gases in Confined Spaces
The hazardous gases that accumulate usually originate in confined spaces themselves or leak in. Some familiar sources are discussed below.
- Decomposition: Decaying plant and animal materials produce methane and hydrogen sulfide. These gases are toxic and flammable.
- Respiration: When people, animals, and grains respire in poorly ventilated confined spaces, the oxygen is consumed and carbon dioxide is produced, creating an oxygen-deficient atmosphere.
- Oxidation: The reaction of materials and substances with oxygen during oxidation will also consume atmospheric oxygen, potentially reducing it to dangerous low levels.
- Chemical reactions: Several stored chemicals either decompose or react with air or other substances, producing gases that are toxic, flammable, or both. Some chemicals can be highly reactive and corrosive, which can deteriorate living substances and other substances.
- Work processes and equipment: Several jobs, like welding, cutting, painting, and cleaning, produce toxic fumes or volatile organic compounds (VOCs) that are toxic or flammable. Equipment like tanks, valves, or pipes that leak can release hazardous gases that they contain into confined spaces.
- Environmental conditions: Changes in temperature and pressure can accelerate chemical reactions or cause evaporation of volatile substances and liquids.
- External seepage into confined spaces: Hazardous gases produced outside confined spaces can seep into them through air, soil, pipelines, and other means.
The location where gases are accumulated depends on their properties. Lighter gases gather in higher areas, while gases heavier than air sink to the bottom of confined spaces. Understanding the work processes and situation can help determine the gases produced and the areas of accumulation, thereby identifying risks for workers entering confined spaces.
The dangers associated with gases in confined spaces vary by industry, as illustrated in Figure 1. The construction industry has the highest number of suffocation accidents in confined spaces, followed by the manufacturing and service industries.
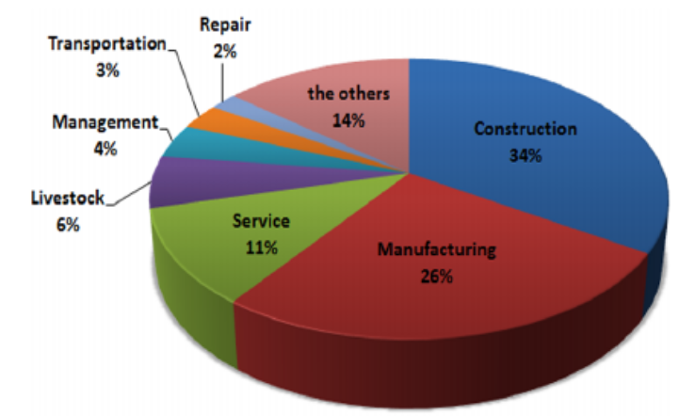
Figure 1: Suffocation accidents occurring in confined spaces in different industries, Lee et al (2016). (Image credits: https://doi.org/10.15269/JKSOEH.2016.26.4.436)
Hazardous Gases in Confined Spaces
The three leading causes of fatalities in confined spaces over the decades have been oxygen deficiency, hydrogen sulfide, and carbon monoxide. The percentage changes, and the number one cause can change, but these three causes remain the top confined space risks. For example, in 1980-1989, oxygen deficiency caused 60% deaths, but in 2012, CO was the leading risk.
The other gases found in confined spaces include carbon dioxide, ammonia, chlorine, methane, propane, hydrogen cyanide, sulfur dioxide, VOCs, and others. Safety managers should evaluate risks associated with these gases based on their respective industries.
Carbon Monoxide
Carbon monoxide (CO) is produced due to incomplete fossil fuel combustion in engines, tools, and heaters. It is colorless, odorless, tasteless, non-irritant, and challenging to detect without the use of gas analyzers. It can cause dizziness and headaches, loss of consciousness, and death due to hypoxia, as it replaces oxygen in the bloodstream. The gas is toxic at concentrations of 35 ppm over an 8-hour workday. It is slightly heavier than air but disperses everywhere. The gas is not industry-specific, as it originates from fossil fuels that are commonly used as fuels in most places. Avoiding fossil fuel use in confined spaces and ensuring good ventilation can prevent its buildup.
Hydrogen Sulfide
Hydrogen sulfide (H2S) is produced from decaying organic matter and is found in various industries, including agriculture, wastewater treatment, sewage systems, maintenance holes, and petroleum refineries. It has an odor similar to rotten eggs at low concentrations; at high concentrations of 100 ppm, it causes paralysis of smell. It is a heavy gas that tends to accumulate near the floor. It is toxic and flammable even at very low concentrations. It is an irritant even at 10 ppm and lethal over 500 ppm. Lower levels irritate the skin, eyes, and the respiratory system. Using personal protective equipment (PPE) is required when entering confined spaces that may contain hydrogen sulfide (H2S).
Oxygen
Usual oxygen (O2) levels in the air are 20.9 %. Levels below 19.5% are dangerous. Oxygen depletion can occur in silos and other industries due to the processes of rusting, combustion, welding, and the decomposition of organic matter. Low levels of oxygen can impair judgment, cause a lack of coordination, dizziness, fatigue, unconsciousness, and death. Though 19.5% is the danger level, people should not enter confined spaces with less than the normal levels of the gas.
Higher concentrations of oxygen, above 23.5%, can be a safety hazard as they increase the risk of fires and explosions.
Carbon Dioxide
Carbon dioxide (CO2) is indirectly hazardous as it accumulates and displaces oxygen. It occurs in places where oxygen is used up rapidly, resulting in oxygen depletion. Levels above 1% are dangerous and a serious health risk.
Ammonia
Ammonia (NH3) is produced in farms due to manure decomposition in manure pits or closed animal pens. It is also formed in fertilizer storage facilities. It has a characteristic odor and is lighter than air. Concentrations of 20-50 ppm can irritate the eyes, skin, and respiratory tract. Prolonged exposure causes severe respiratory issues.
Chlorine
Chlorine (Cl2) gas is used as a disinfectant in water treatment and as a cleaning agent. It is yellowish-green in color and has a pungent odor. It is released by cleaning agents, such as bleach, in confined spaces. Chlorine is a potent irritant that affects the eyes, skin, and respiratory system. It causes coughing, respiratory issues, and lung damage. Proper ventilation and PPE for workers are necessary.
Nitrogen Dioxide
Nitrogen dioxide (NO2) is produced through the combustion of fossil fuels, welding, and chemical reactions, and accumulates in confined spaces. Short-term exposure causes respiratory irritation, wheezing, and shortness of breath. While more prolonged exposure causes lung damage and diseases. Higher concentrations can lead to death. OSHA has set 5 ppm as the permissible level.
Hydrogen Cyanide
Hydrogen cyanide (HCN) is a volatile inorganic compound (VIC). It is an acid, and a liquid at room temperature, but it is a gas above 78°F. It is a precursor to many chemicals, used in the extraction of gold and silver from ores, and as a fumigant. The gas has a slight almond-like smell, is lighter than air, and tends to accumulate near the roof. HCN levels can increase in confined spaces, posing a health and safety risk due to their toxicity and flammability. HCN affects the respiratory, cardiac, and neurological systems, causing dizziness, irregular heartbeat, seizures, and instant death.
Volatile Organic Chemical
Volatile organic compounds (VOCs) are commonly found in confined spaces within the oil and petrochemical industries. VOCs are heavier than air and tend to accumulate near the floor. VOCs displace oxygen, creating an oxygen-deficient atmosphere that can lead to suffocation and death.
Methane:
Methane (CH4) is formed due to the decomposition of organic matter in sewers, wastewater treatment plants, manure pits, chemical, and fuel storage tanks. It is lighter than air and rises, often found below the lids of confined spaces. It is non-toxic but can displace oxygen to cause asphyxiation. The most significant risk, however, is its flammability.
Propane
Propane (C3H8) can accumulate in confined spaces due to accidental release from adjacent structures. It is a colorless, odorless gas. It poses a confined space hazard due to its high flammability and the potential to form explosive mixtures with air at room temperature. It is not toxic, but it can displace oxygen, creating an oxygen-deficient atmosphere. As oxygen levels fall, people suffer from nausea, convulsions, coma, and death.
Some other toxic gases that can be present are phosgene, freon, and acidic gases (hydrochloric and sulfur dioxide).
Limiting Confined Space Risks
One of the most crucial methods for limiting accidents in confined spaces is monitoring air quality, as recommended by OSHA. The air can be monitored continuously through fixed gas analyzers. However, testing the air in confined spaces with portable devices before entry is necessary. Since gas distributions are not even, several areas should be tested. Maintain ventilation, provide training, and PPE as needed if the risk assessment identifies the presence of toxic gases.
Interscan provides fixed and portable gas analyzers for a range of gases commonly found in confined spaces, including oxygen, carbon monoxide, ammonia, chlorine, hydrogen sulfide, nitrogen dioxide, hydrochloric acid, hydrogen cyanide, and sulfur dioxide.
Contact us to learn more about our products for monitoring air quality in confined spaces.
Sources
Henderson, B. (2023, June 4). What combustible gases are associated with confined spaces? Retrieved from https://www.safeopedia.com/what-combustible-gases-are-associated-with-confined-spaces/7/7114
Lee, Jung Wan, Kim, Tae Hyeung, Ha, Hyun Chul, Piao, Cheng Xu, & Ahn, Kwangseog. (2016). Analysis of Suffocating Accidents in Confined Spaces in the Past 10 Years (2005-2015). Journal of Korean Society of Occupational and Environmental Hygiene, 26(4), 436–444. https://doi.org/10.15269/JKSOEH.2016.26.4.436
Selman, J., Spickett, J., Jansz, J., & Mullins, B. (2018). An investigation into the rate and mechanism of incident of work-related confined space fatalities. Safety Science, 109, 333-343. https://doi.org/10.1016/j.ssci.2018.06.014
Smith, B. (2006, Nov 1). Confined Spaces and Gas Detection. Retrieved from https://ohsonline.com/articles/2006/11/confined-spaces-and-gas-detection.aspx?Page=1
Yan, L., Yantek, D. S., DeGennaro, C. R., & Fernando, R. D. (2023). Mathematical Modeling for Carbon Dioxide Level Within Confined Spaces. ASCE-ASME Journal of Risk and Uncertainty in Engineering Systems, Part B: Mechanical Engineering, 9(2), 024501.


