- Four gases, carbon monoxide, ammonia, hydrogen sulfide, and chlorine, are the top causes of chemical injuries, deaths, and evacuations in industries.
- The effects occurred due to exposure to the individual gases.
- Carbon monoxide and ammonia are released due to faulty equipment, while chlorine is released due to human error.
- Hydrogen sulfide deaths mainly occurred in confined spaces.
According to the World Health Organization/International Labour Organization (WHO/ILO) Joint Estimates published in 2021, occupational gases and fumes were the second most significant factor in causing global occupational deaths. The 450,381 deaths due to gases occur annually due to chronic obstructive pulmonary disease. Workers face occupational exposure to hazardous gases that cause diseases, cancers, poisonings, or fatal injuries in fires and explosions. These injuries, illnesses, and deaths are preventable if companies follow known and established procedures to prevent and limit exposure. The first step is risk identification. This article discusses several gases that are among the top occupational hazards across various industries.
Sources of Hazardous Gases
Gas is any substance that has no definite shape or volume and is volatile at certain temperatures and pressures. Gases are produced from heating solids, evaporation from liquids, and as a product of chemical reactions in the industries. Incomplete combustion can release carbon monoxide. Natural decomposition processes in wastewater, manure, and textile or pulp effluents can generate hydrogen sulfide. Welding and chemical manufacturing produce ammonia and chlorine. While fuels, which are organic compounds like propane, can leak to cause fires and explosions.
Fumes are formed from industrial and chemical processes, such as welding and metal forming. The gases and fumes create hazardous atmospheres, as many are colorless and odorless, mainly due to accumulation in poorly ventilated areas and confined spaces. Fumes can be irritants or toxic. Mists are water droplets in the air that carry chemicals, which settle on floors and windows. They can be harmful if inhaled.
Another source of hazardous gases is the use of fumigants to control insects, rodents, and microbes. Docks, freight, containers, and storage facilities can have these gases. The three common ones are
- Methyl bromide (bromomethane) is used to treat soil and timber.
- Aluminum phosphide (phosphine) is used to fumigate food, tobacco, and perishables.
- Hydrogen cyanide is not commonly used due to the considerable danger it poses, but it can be a substitute for phosphine and bromomethane.
Gases, fumes, and mists are commonly found in industrial work areas, storage rooms, containers or cargo holds, vehicle garages, mines, and refineries.
Table 1: Examples of inorganic gases in the workplace. (Credits: https://oshwiki.osha.europa.eu/en/themes/gases)
| Gas | Hazard | Principal usage/occurrence |
| Ammonia | Toxic/flammable | Fertilizers or from bacterial action on organic material, refrigeration (see, for example, [16]), synthesis |
| Arsine | Toxic/flammable | Electronics, synthesis |
| Carbon dioxide | Toxic/asphyxiant | Refrigeration, beverages, synthesis, combustion products |
| Carbon monoxide | Toxic/flammable | Synthesis, fuels, combustion products |
| Chlorine | Toxic | Synthesis, food processing, water treatment |
| Fluorine | Toxic | Synthesis, water treatment |
| Hydrogen chloride | Toxic | Synthesis, chemicals, and food processing |
| Hydrogen cyanide | Toxic | Synthesis |
| Hydrogen fluoride | Toxic | Synthesis, aluminum production, metals/glass processing |
| Hydrogen sulfide | Toxic | Chemical processing, oil by-product, bacterial action by-product |
| Mercury | Toxic | Electrical/electronics, chemical manufacturing |
| Nickel carbonyl | Toxic/flammable | Nickel processing |
| Nitrogen monoxide | Toxic | Nitric acid synthesis, process, and combustion product, medical |
| Nitrogen dioxide | Toxic | Nitric acid, fertilizers, explosives, synthesis, materials |
| Nitrous oxide | Toxic/asphyxiant | Anesthetic, specialist fuel |
| Ozone | Toxic | Water treatment, bleaching |
| Phosgene | Toxic | Synthesis, by-product of the chlorinated hydrocarbons processing |
| Phosphine | Toxic/flammable | Electronics, synthesis |
| Radon | Toxic/radioactive | Medicine, naturally occurring |
| Silane | Toxic/flammable | Electronics |
| Stibine | Toxic/flammable | Fumigation, electronics |
| Sulphur dioxide | Toxic | Synthesis, bleaching, food, fertilizers, and catalyst |
Hazardous gases and fumes can be inorganic or organic compounds.
- Inorganic gases can be simple elements, such as chlorine (Cl2) or fluorine (F), or compounds like ammonia (NH3) or carbon monoxide (CO). See Table 1 for a list of occupational inorganic gas hazards.
- Organic gases are compounds that have carbon and hydrogen as their basic components, for example, ethylene (C2H4). They can also have other elements in the compound, such as oxygen (as in formaldehyde, HCHO), or nitrogen (as in hydrazine, CH3NHNH2). Many organic gases are produced in the petrochemical and chemical industries and are toxic, flammable, and pose asphyxiation risks.
Hazardous Nature of Gases
Gases are hazardous and pose an occupational risk because some can be toxic, reactive, corrosive, flammable, oxidizing, or asphyxiating. A few gases can have more than one adverse effect. According to PennEHRS (Environmental Health and Radiation Safety), the hazardous qualities are defined as follows:
- Toxic gases are those in which 50% of the sample population of rats dies due to exposure to a target gas. The value is called LC50. Poisonous gases that produce LC50 at 200 to 2000 ppm are classified under acute Toxicity GHS Category 2. Highly toxic gases are those that have an LC50 of less than 200 ppm and are classified under the Acute Toxicity GHS Category 1. Examples include hydrogen sulfide and ethylene oxide.
- Corrosive Gases are very reactive and affect metals, substances, and living tissue. The magnitude of the effect depends on the solubility of the gas in question. Ammonia and hydrochloride cause more severe symptoms than less soluble gases, such as nitrogen dioxide, sulfur dioxide, and phosgene.
- Flammable and pyrophoric gases are substances that are gases at 20°C (68°F) and an absolute pressure of 14.7 psi. The gas has to be between its upper flammable limit (UFL) and lower flammable limit (LFL). For example, hydrogen is flammable when present at concentrations between 4% and 75% of the air volume. Oxygen and an ignition source are necessary to initiate burning. Pyrophoric gases self-ignite without an external spark or flame at or below 130°F.
- Dangerously reactive gases are compressed and unstable. A slight increase in temperature, pressure, or mechanical shock can trigger reactions that result in explosions.
- Oxidizing gases are compounds that contain oxygen at a concentration higher than the normal 21% concentration in air, which can catalyze fires and increase the likelihood of explosions—for example, halogen gases and nitrogen oxides.
- Asphyxiating gases may not be hazardous in themselves, but their accumulation in poorly ventilated and confined spaces reduces oxygen levels below normal, suffocating anyone entering these oxygen-depleted environments. However, chemical asphyxiating gases like carbon monoxide and hydrogen sulfide are also reactive and hazardous, or, like methane, are flammable.
Identifying and understanding the properties of gases that cause injuries can help reduce morbidity, evacuations, and damage to infrastructure.
Most Common Hazardous Gases
Several industry-specific gases are emitted. However, some common gases occur in many industries. According to a 2015 CDC report, of the 57,975 chemical release incidents reported to the Hazardous Substances Emergency Events Surveillance (HSEES) system between 1999 and 2008, 95% occurred due to exposure to a specific chemical. Among them were three gases. The five chemicals were responsible for 37% (4924 cases) of injuries, even though they accounted for only 3% (1383 cases) of release incidents. These five caused a quarter of all deaths attributed to chemicals. These five chemicals were the gases carbon monoxide, ammonia, and chlorine, as well as hydrochloric acid and sulfuric acid.
- Around 70-90% of the releases occurred in fixed facilities. Hydrochloric and sulfuric acid releases were also high during transport (30% and 15%, respectively).
- Equipment failure was the leading cause of chemical releases, accounting for 46% of ammonia, 45% of carbon monoxide, and 41% of sulfuric acid releases.
- Human error was the leading cause in the cases of chlorine (37%) and hydrochloric acid (41%).
Table 2: “Number of incidents, persons injured, evacuations, and shelter-in-place orders, by top five chemicals released — Hazardous Substances Emergency Events Surveillance system, nine states, * 1999–2008,” Anderson (2015). (Credits: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6402a6.htm#Tab1)
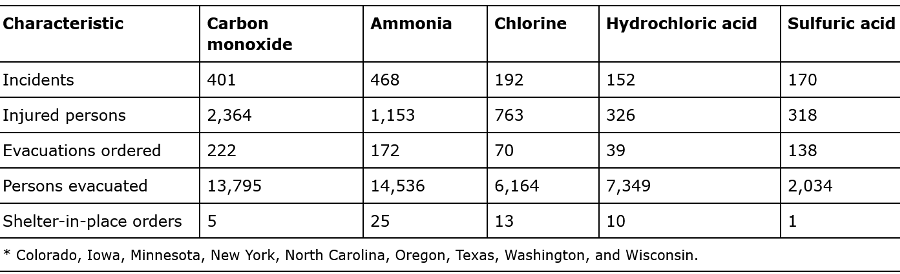
The three gases were the top causes of injuries and deaths due to chemicals, as shown in Table 2.
- Carbon monoxide affected the most people (2,364) and resulted in the highest number of evacuations (222). CO was the chemical that resulted in the most deaths among the injured (3%). The industries involved in CO releases include salons, auto repair shops, and religious organizations. However, most of the CO releases were at private residences.
- Ammonia resulted in 1,153 injuries, and the largest number of people being evacuated. Half of the ammonia-related injuries happened in food processing and agriculture, where the gas is used as a refrigerant. The other units where ammonia-related injuries occurred were the textile and apparel manufacturing industries.
- Chlorine is not among the top ten chemicals released, but it caused 763 reported injuries. It caused the fewest number of fatalities. Most of the chlorine releases occurred in facilities producing lumber, paper, printing chemicals, leather, petroleum, and stone. Chlorine is a vital component used in the manufacture of a wide range of products, including silicones, foams, and plastics. It is also used as a disinfectant for water, pulp, and paper bleaching.
- Hydrogen sulfide is identified as the gas that causes the second-highest number of fatalities due to a single inhalation, primarily occurring in confined spaces, in 2017, according to the Bureau of Labor Statistics. The gas is produced due to the decomposition of organic material.
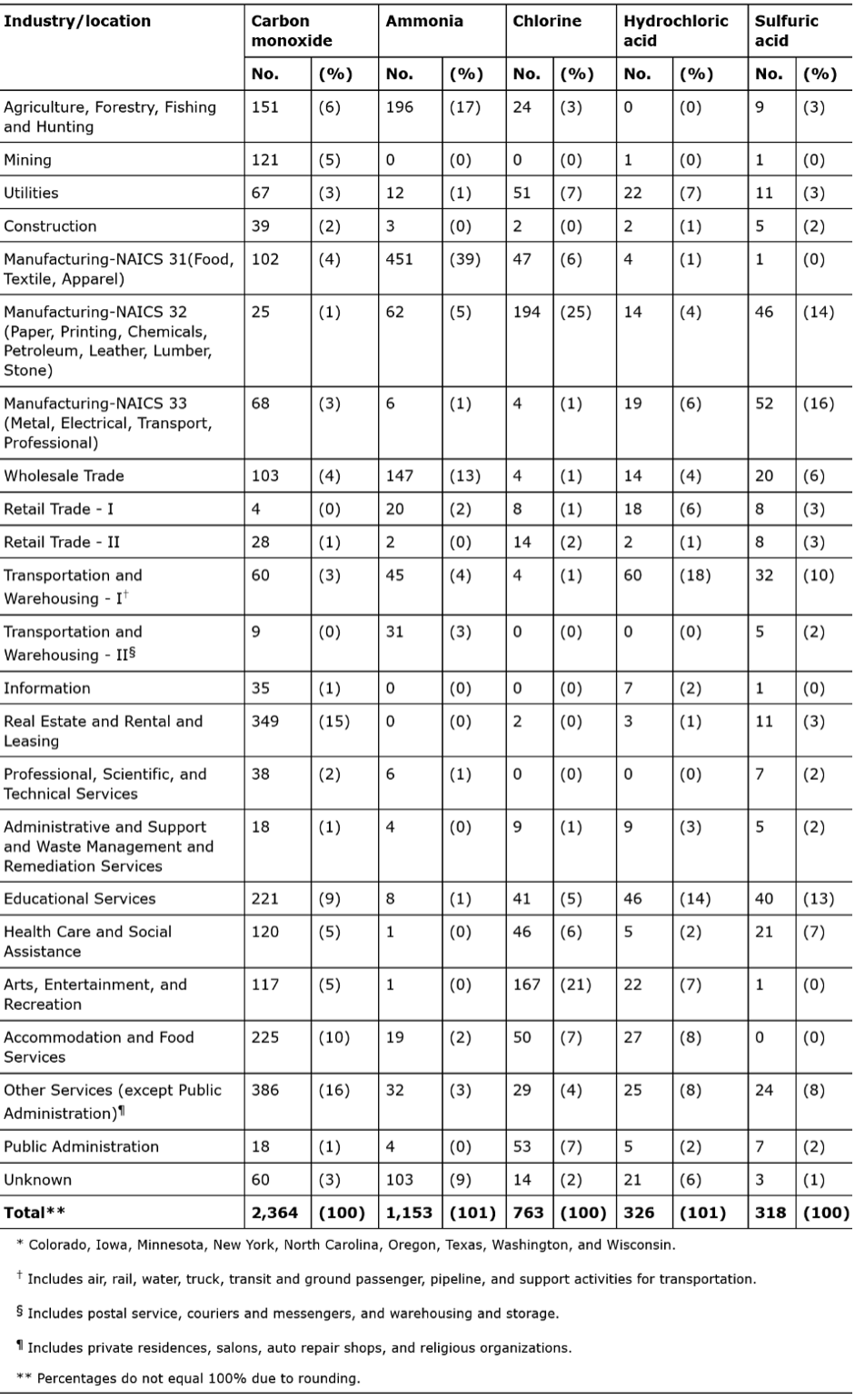
Carbon monoxide and ammonia are responsible for the highest number of injuries, deaths, and evacuations. The industries where most accidental releases of gases occur are listed in Table 3. Agriculture, hunting, forestry, fishing, mining, utilities, construction, and manufacturing are some industries where these gases are found.
TABLE 3. “Number and percentage of injured persons in incidents with top five chemicals released, by industry/location — Hazardous Substances Emergency Events Surveillance system, nine states,* 1999–2008,”Anderson (2015). (Credits: https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6402a6.htm#Tab1)
Preventing Gas Hazards
Besides the four gases listed above, safety managers and industrial hygienists should be aware of the industry-specific emissions to ensure the safety of staff and infrastructure. The managers should identify the gases that their facilities use or emit. Then, using the hierarchy of controls, they should attempt to eliminate or minimize the risks associated with the hazardous gases. Monitoring industrial facilities around the clock through strategically placed gas detectors can ensure gas levels stay below permitted limits or alert staff in the event of any increase in concentrations. Portable sensors are helpful to check gas levels in confined spaces and poorly ventilated work areas before entry. Interscan manufactures fixed and portable sensors to detect over 20 common gases found in various industries.
Contact us at Interscan to find out how we can help keep your staff and facilities safe.
Sources
Anderson, A.R. (2015). Top Five Chemicals Resulting in Injuries from Acute Chemical Incidents — Hazardous Substances Emergency Events Surveillance, Nine States, 1999–2008. Retrieved from https://www.cdc.gov/mmwr/preview/mmwrhtml/ss6402a6.htm#Tab1)
Bureau of Labor Statistics, U.S. Department of Labor, The Economics Daily, Fatal chemical inhalations in the workplace up in 2017 at https://www.bls.gov/opub/ted/2019/fatal-chemical-inhalations-in-the-workplace-up-in-2017.htm (visited August 25, 2025).
Cornell Education. (n.d.). 16.4.5 Hazards of Specific Gases. Retrieved from
Lambrini, K., Christos, I., Petros, O., & Alexandros, M. (2018). Dangerous Gases and Poisoning: A. Archivos De Medicina, 3(2), 26.
OSHWIKI. (2016, March 1). Gases. Retrieved from https://oshwiki.osha.europa.eu/en/themes/gases
PennEHRS. (2024, April 12). SOP: Hazardous and Highly Toxic Gases. Retrieved from https://ehrs.upenn.edu/health-safety/lab-safety/chemical-hygiene-plan/standard-operating-procedures/sop-hazardous-and
Warren, P. J. (1997). Hazardous gases and fumes. Butterworth-Heinemann,
WHO/ILO. (2021). WHO/ILO Joint Estimates of the Work-related Burden of Disease and Injury, 2000–2016. Retrieved from https://www.ilo.org/sites/default/files/wcmsp5/groups/public/@ed_dialogue/@lab_admin/documents/publication/wcms_819788.pdf


