- Hydrogen sulfide is toxic, flammable, and corrosive, and must be monitored in industries that use or generate it.
- The industries most at risk of hydrogen sulfide emissions are oil and gas, paper and pulp, chemical manufacturing, food processing, and solid and wastewater treatment.
- Monitoring air in affected industrial facilities for H2S emissions, whether through normal processes or accidents, is one of the most crucial steps in ensuring the safety and health of workers.
Hydrogen sulfide is a recognized and common occupational hazard found in many industries. It puts workers at risk and causes human and financial losses. The gas is unhealthy at even low levels and is capable of instantly killing people when present at concentrations of 1000-2000 ppm. Safety managers and industrial hygienists, find out if your facility is at risk from hydrogen sulfide and if you need to monitor this dangerous gas.
Hydrogen Sulfide (H2S) Gas
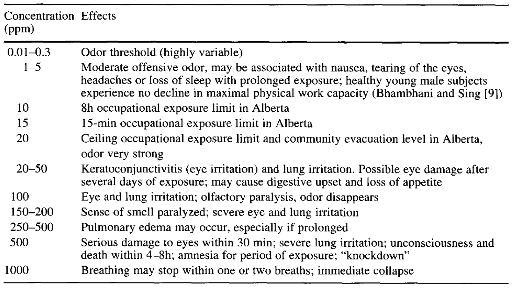
Table 1: Health effects of H2S at various exposure levels, Guidotti, 1994. (Credits: https://www.csun.edu/~dorsogna/byron/Ortona/alberta.pdf)
Hydrogen sulfide (H2S) is a gas at standard temperature and pressure. It is colorless and, at low concentrations, smells of rotten eggs. At 100 ppm, when the gas is very toxic, it causes smell paralysis and cannot be detected by its odor. So, people can enter areas unaware of its presence and often be overtaken by the effects of the gas. It is heavier than air and accumulates in low-lying and poorly ventilated areas, like confined spaces.
H2S is harmful because it is flammable, corrosive, and toxic:
- Fire hazard: The gas is flammable and a fire hazard at concentrations of 4-44% in the air, and can ignite due to a spark or open flames. It burns with a blue flame, and as the temperature increases, the intensity of the fire does too. Since the gas is heavy, it does not disperse up in the air but gets transported far from its source. If the gas encounters a flame, the fire spreads far.
- Corrosive: When H2S dissolves in water, it can become corrosive, destroying pipes, equipment, and infrastructure, leading to potential hazards.
- Poisonous: Hydrogen sulfide is toxic even at low concentrations and, with acute exposure, can lead to eye and respiratory tract irritation. The gas causes death at 100 ppm, and is instantly fatal at 1000 ppm. See Table 1 for the health effects of H2S.
Hydrogen Sulfide Monitoring
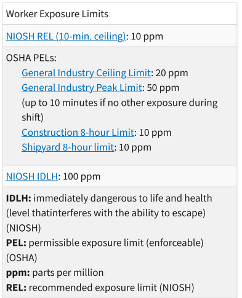
Given the high safety and health risks that H2S poses, in the USA, there are strict national standards to limit its levels to extremely low parts per million (ppm) in workplaces, safeguarding workers’ health. These standards have been set by the Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH); see Table 2 for details.
Table 2: Permissible limits according to OSHA and NIOSH. (Credits: https://www.osha.gov/hydrogen-sulfide/hazards)
H2S is produced as a byproduct of the decomposition of organic matter and rocks that contain sulfur in industrial and natural processes. The gas can also occur in industries because it is used as a chemical reagent in several sectors. Industries at risk from H2S should monitor the air for the gas to detect when levels rise above permissible limits, and take corrective measures or evacuate the space promptly.
The industries where H2S is commonly found and should be monitored are oil and gas, paper and pulp, chemical manufacturing, food processing, plastic and rubber production, sewage and wastewater treatment, solid waste treatment, mining, agriculture, tanning, etc.; see Figure 1.
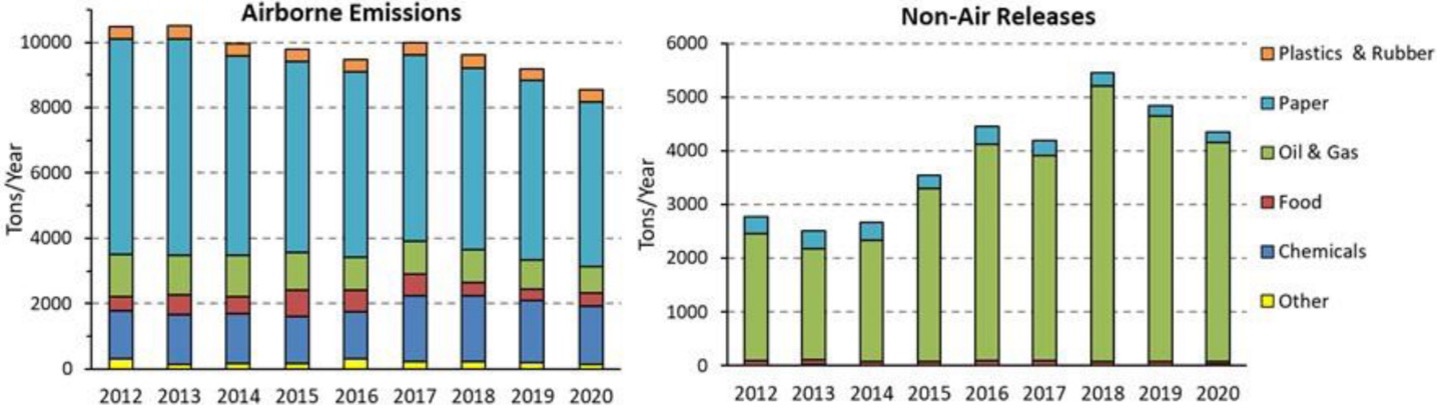
Figure 1: “H2S emissions reported in the U.S. Toxics Release Inventory (TRI) to air and other media by the top emitting industry sectors, 2012–2020. “Oil & gas” includes releases from petroleum refineries, bulk plants and terminals,; “Paper” includes pulp mills; “Other” includes primary metals, nonmetallic mineral products, water, wastewater, beverages, electric utilities, hazardous waste, wood products, leather, poultry and egg production, machinery, furniture, transportation equipment, and metal mining,” Batterman et al 2023 (Image credits: doi.org/10.1080/10408444.2023.2229925)
-
Oil and Gas Industry
H2S is produced during the mining, refining, and processing of natural gas and petroleum, and a couple of deaths annually due to the gas are reported from Alberta, Canada.
Mining: H2S forms naturally and is one of the most hazardous gases present in mines, primarily due to its heaviness and tendency to accumulate in low-lying areas. Air in mines must be checked before entry.
Refining: The petroleum products contain sulfur that is removed during refining through a reaction with hydrogen, producing the gas, making them the foremost man-made source of H2S.
Processing: H2S is also found naturally as sour gas in natural gas. H2S is released when it is removed before distribution. H2S monitoring is also required during the transportation and storage of oil and gas.
Multiple fixed sensors at various points, along with portable detectors, are necessary to protect workers.
-
Pulp and Paper Industry
H2S is generated in the pulp and paper industries during the kraft process and treatment of effluents of the industry.
Production: The Kraft process, which uses sulfates, is the most common pulping method in the world. It is used to separate and obtain fibers from wood using white liquor or chemicals such as
sodium hydroxide and sodium sulphate. The chemical processing during bark combustion,
wood-handling, pulp cooking and washing, bleaching, and evaporation, releases various gases, like methyl mercaptan, dimethyl, including H2S, which has caused fatalities. The sulphite process also releases the gas, but to a lesser extent. H2S is also produced in recycled paper mills.
Effluents: H2S is present in the wastewater due to losses from the liquor cycle. Also, the decomposition of organic material in the effluents produces H2S that must be monitored.
Odor is one of the main complaints for workers and the neighborhood.
-
Manufacturing Factories
Hydrogen sulfide is used as a chemical reagent to make heavy water, cut oils, lubricants, and in metallurgy. It is the primary material to produce sulfuric acid and sulfur through oxidation in the Claus process. These products are used in manufacturing pesticides, pharmaceutical drugs, and leather. Sulfuric acid is a vital base substance in the chemical industry.
Using sensors to maintain gas levels can protect workers and equipment.
-
Food Processing Facilities
H2S is used in the food processing industry as a gas or liquid. Small amounts of the gas act as a signaling molecule that alleviates oxidative stress by increasing antioxidant activity and maintaining cell membrane integrity. It lowers respiration and ethylene activity and delays the ripening and decaying of fresh produce. The gas keeps food (fresh produce and meat) safe by acting as a biocide against mollusks, insects, and rodents; a fungicide to control molds and fungi; and a disinfectant to prevent bacterial growth. As a result, the food retains better qualities such as color, odor, texture, taste, and aroma.
H2S is used as a reference odor to evaluate off-flavors in quality control. The gas can also be produced in the waste treatment of the food processing industry.
Using gas sensors to monitor H2S production not only enhances food quality but also keeps workers safe.
5. Rubber and Plastics Industries
Natural rubber latex production processes can produce H2S in low concentrations. Chronic exposure can cause eye irritation. Moreover, H2S is also created from the effluents of the rubber industry in higher concentrations that are harmful to people working in the units. Monitoring the air in these areas is recommended.
H2S gas is also formed in landfills and recycling units that contain plastics. The gas is produced as a byproduct when sulfate-reducing bacteria degrade organic additives from plastic resins. The source is not plastic polymers, but the plasticized polyvinyl chloride (PVC).
6. Sewage and Wastewater Treatment Plants
H2S and its odor are strongly associated with sewage systems and wastewater treatment plants, as the gas is produced due to the decomposing organic matter present.
Sewers: H2S produced in confined and underground sewer and drainage systems, as well as in manholes, has caused numerous fatalities and is a significant source of concern. Portable gas detectors are a must in these situations.
Waste treatment plants: Areas such as septic tanks and wastewater treatment plants also generate significant amounts of H2S, which can be monitored through fixed and portable devices.
-
Indoor Solid Waste Management
Landfills also have decomposing organic matter and produce H2S. Most landfills are open, and the H2S produced escapes and disperses in the open air. However, closed landfills and solid waste treatment centers, especially those that use biological and mechanical methods, can accumulate H2S gas in high concentrations. The air in indoor solid waste management systems must be monitored for H2S gas, and ventilation can be used to reduce its levels.
8. Mining Processes
H2S detection is necessary in all mines where sulfur compounds are found in the soil.
H2S gas is produced in high-sulfur coal mines, though to a lesser extent than in natural gas and oil fields. Information on sources of H2S is not well established. Still, extrapolating from known processes in the oil and gas industry, it could be formed during coal formation through bacterial action during peat accumulation and diagenesis (physical, biological, and chemical processes that occur during rock formation).
In other mines, iron pyrite decomposition can produce H2S that is dangerous even in low concentrations in confined mines.
-
Agriculture
Hydrogen sulfide is formed on farms in liquid manure storage tanks and pits, agricultural biogas systems, silos, animal stalls, and barns. Workers handling, mixing, or cleaning these facilities can be exposed to the gas that accumulates due to less ventilation. In confined spaces like silos and tanks, it can displace oxygen and lead to suffocation in addition to the health effects the gas has. Biogas generators for producing renewable fuel produce H2S in places used for storing input materials and outputs, and anaerobic digester equipment for producing biogas.
Portable, handheld gas detectors can save lives and protect buildings in the industry by eliminating the health and fire hazards associated with H2S.
-
Tanning
Several locations in a tannery can release H2S. These are detailed below:
Storage: When acids are stored with sodium sulfide, H2S can be formed through accidental spills and poor handling.
Pretanning area: When acids are added to drums, pits, or paddles during leather liming, deliming, and pickling, H2S is formed.
Re-tanning: During retanning, the use of sulfur, sulphates, or liquors containing sulfide or acids can release H2S.
Effluent treatment, storage, and discharge lines: The effluent has high organic matter content and can produce H2S. When the solids content increases due to dewatering and sludge thickening, and liquor is mixed, more H2S is produced.
Empty pits: Even unused pits can contain H2S gas if they are not cleaned thoroughly after use.
Since many areas in tanning involve small confined spaces, portable gas detectors should be used to check for H2S gas levels before beginning work.
Monitoring H2S
Gas detectors are used to monitor H2S. Monitoring is done in two ways: fixed sensors and portable devices. The sensors should be able to track H2S emissions in ppm concentrations and have audio and visual alarms to alert personnel of higher-than-permissible levels of the gas. Electrochemical sensors are commonly used to detect H2S.
- Fixed gas detectors: Several sensors are placed at spots in areas at risk of H2S gas leakages or production to monitor the air 24/7. Some gas detectors have data logging capacities to provide records of emission levels necessary for treating health effects or tracing the cause of leaks.
- Portable devices: In high-risk areas and confined spaces, workers must also use portable gas detectors to check the air for H2S before entering.
Interscan produces fixed and portable hydrogen sulfide detectors that can be used in various industries at the ppm levels required to monitor air and reduce the risk of H2S exposure.
Besides monitoring the air, several other measures must be used to protect workers. These include the use of H2S scrubbers, ventilation, engineering controls, etc., which can remove H2S from the air. Safe work practices and use of personal protective equipment with artificial respirator support in confined spaces can also reduce exposure when H2S levels cannot be immediately lowered.
Find out more about Interscan’s portable GASD8000 and fixed AccuSafe for your H2S monitoring needs.
Sources
Batterman, S., Grant-Alfieri, A., & Seo, S. H. (2023). Low level exposure to hydrogen sulfide: a review of emissions, community exposure, health effects, and exposure guidelines. Critical reviews in toxicology, 53(4), 244–295. https://doi.org/10.1080/10408444.2023.2229925
Guidotti, T. L. (1994). Occupational exposure to hydrogen sulfide in the sour gas industry: some unresolved issues. International archives of occupational and environmental health, 66(3), 153-160.
IIR.Umweltbundesamt.de. (2024, Nov 6). 2.H.1 – Pulp and Paper Industry. Retrieved from https://iir.umweltbundesamt.de/2023/sector/ippu/pulp_paper_food/pulp_and_paper_industry/start
Kioumars Taheri* and Farhad Mohammad Torab. (2023). Investigating the Application and Role of H2S Gas Detectors in the Safety of Mines and Oil, Gas and Petrochemical Industries.
Progress Petrochem Sci. 6(1). PPS. 000626. 2023. DOI: 10.31031/PPS.2023.06.000626
Lim L.Y., Bong C., Ho W.S., Hashim H., Taib M.R., Ng P.S., Wong K.Y., 2022, Field Measurement and Dispersion Modelling of Hydrogen Sulphide for Recycled Paper Mill in Malaysia. Chemical Engineering Transactions, 97, 499-504. DOI:10.3303/CET2297084
New Jersey Department of Health. (2012, May). Hazardous substance- Hydrogen sulfide. Retrieved from https://nj.gov/health/eoh/rtkweb/documents/fs/1017.pdf
Nunes, M., Kalinowski, C., Godoi, A., Gomes, A., & Cerqueira, M. (2021). Hydrogen sulfide levels in the ambient air of municipal solid waste management facilities: A case study in Portugal. Case Studies in Chemical and Environmental Engineering, 4, 100152. https://doi.org/10.1016/j.cscee.2021.100152
Ontario. (2022, October). Hydrogen sulphide in agricultural biogas systems. Retrieved from https://www.ontario.ca/page/hydrogen-sulphide-agricultural-biogas-systems
OSHA. (n.d.). Hydrogen Sulfide- Evaluating and Controlling Exposure. Retrieved from
https://www.osha.gov/hydrogen-sulfide/evaluating-controlling-exposure
Siddiqui, M. W., Deshi, V., Irfan, M., Kumar, V., Homa, F., Mir, H., & Singh, D. R. (2023). Hydrogen sulfide: Promising applications for postharvest quality improvement of fruit and vegetables. Postharvest Biology and Technology, 202, 112394. https://doi.org/10.1016/j.postharvbio.2023.112394
Tsuchida, D., Kajihara, Y., Shimidzu, N., Hamamura, K., & Nagase, M. (2011). Hydrogen sulfide production by sulfate-reducing bacteria utilizing additives eluted from plastic resins. Waste management & research: the journal of the International Solid Wastes and Public Cleansing Association, ISWA, 29(6), 594–601. https://doi.org/10.1177/0734242X10388556
Uddin, N., Tamanna, T. R., khan Panni, M. F. A., & Hossain, M. I. (2023). Risk assessment of hydrogen sulfide (H₂S) gas and its impact on human health: evidence from tannery industry of a developing country. European Journal of Engineering and Technology Research, 8(6), 31-36.
Xu, L., Yang, K., Chen, L., Liu, L., Jiang, Z., Zhu, J., & Wu, H. (2023). Analysis of the mechanism for H2S generation and the main factors controlling gas production in coal seams. Fuel, 343, 127935. https://doi.org/10.1016/j.fuel.2023.127935


