- Ammonia gas is hazardous because it is highly toxic and explosive.
- Exposure can cause severe and permanent injury and even death.
- Ammonia detection with multiple fixed and portable detectors to cover the entire fertilizer facility should be part of the safety plan to avoid catastrophes.
Ammonia is a commonly used chemical but is dangerous in concentrated forms. Fertilizer facility engineers need a safety plan that uses reliable ammonia gas detection technology to prevent hazards for the staff and surrounding communities. Find out more about the dangers of ammonia gas and how engineers can use ammonia detection to maintain the safety limits they must keep in fertilizer plants.
Ammonia is Highly Toxic
Ammonia (NH3) is a colorless and pungent gas classified as highly toxic due to its health effects on people. Ammonia can be smelt when concentrations are over 5 ppm.
Ammonia can be released due to accidental leaks in fertilizer factories. Dry ammonia is lighter than air, but because it is hydrophilic (water-loving), it rapidly combines with water or water vapor to form clouds that stay close to the ground, endangering staff. The ammonia clouds can travel several meters outside the fertilizer facilities and affect nearby communities, see Figure 1.

Figure 1: “When ammonia gas is released, it combines with humidity in the air to form a vapor cloud. Even though anhydrous ammonia gas is lighter than air, an ammonia vapor cloud will often stay near the ground, especially in cold weather. This cloud can travel long distances, putting entire communities at risk.” OSHA (Image credits: https://www.osha.gov/sites/default/files/2019-03/fs2-whatsdangerous.pdf)
Ammonia’s hydrophilic nature makes it stick to moist surfaces of a person, like eyes, skin, mouth, throat, and lungs. The health effects ammonia gas can have are described below.
Acute health effects can occur immediately or shortly after contact or inhalation of ammonia gas or contact with liquid ammonia.
- Contact effects are severe eye and skin irritation and eye damage. Contact with liquid ammonia causes frostbite.
- Inhaling ammonia irritates the nose, throat, and lungs, causing coughing, wheezing, and shortness of breath. More exposure causes pulmonary edema.
- Ammonia in 2500-4500 ppm concentrations is fatal in 30 minutes, and over 5000 ppm results in rapid respiratory arrest.
The chronic health effects occur after some time and last for months to years. Repeated exposure causes asthma-like allergies. It can lead to asthma and permanent lung damage. Ammonia’s ability to cause cancer has not been tested so far.
Ammonia is Explosive
Ammonia gas is not flammable, but at concentrations of 15 to 28 percent, the gas is explosive under high heat. Ammonia containers can explode, and the explosion can form poisonous gases like nitrogen oxides (NOx).
In the 2013 West Texas, USA catastrophe, 15 lives were lost in a nitrate explosion in a fertilizer storage plant due to a blast of 7.5-10 tonnes of TNT. The cause was improper monitoring and handling of ammonia.
Explosions harm lives and causes extensive damage to facilities, so there is a need to check and keep ammonia levels low.
Permissible Levels of Ammonia
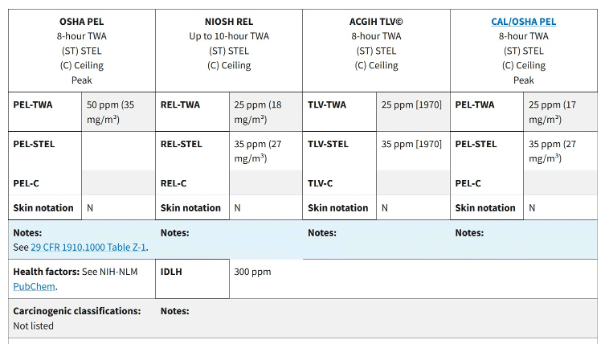
Various agencies in the US have set permissible levels for ammonia due to its hazards.
According to the Occupational Safety and Health Administration (OSHA), the legal permissible exposure limit (PEL) averaged over an 8-hour work shift is 50 ppm.
National Institute for Occupational Safety and Health (NIOSH) limits are as follows:
- The recommended airborne exposure limit (REL) averaged over a 10-hour work shift is 25 ppm.
- The short-term exposure limit (STEL) during any 15-minute work period should not exceed 35 ppm.
American Conference of Governmental Industrial Hygienists (ACGIH) limits are:
- The threshold limit value (TLV) averaged over an 8-hour work shift is 25 ppm.
- STEL during any 15-minute work period should not exceed 35 ppm.
Ammonia is “Immediately dangerous to life or health” (IDLH) at concentrations of 300 ppm.
Though NIOSH and ACGIH standards are more strict, only OSHA limits are legally applicable. However, employers should consider using more stringent standards to protect their staff’s lives.
Table 1: Various limits prescribed by the agencies. (Credit: https://www.osha.gov/chemicaldata/623)
Where Do Ammonia Leaks Occur?
Monitoring the facilities for gas leaks is crucial to all safety planning to ensure fertilizer facilities adhere to and maintain the government regulatory limits to ammonia exposure.
Leaks can occur during transportation, bulk storage, and processing in the various vessels. Ammonia leaks can occur due to equipment malfunctioning, corrosion, and rusting.
Ammonia makes nitrogen fertilizers like urea, ammonium nitrate, urea-ammonium nitrate, ammonium sulfate, and diammonium phosphate. Industrial-grade ammonia is supplied as anhydrous ammonia gas for fertilizer production in the Haber-Oswald process. Fertilizer factories are large with complex machinery, equipment, tanks, pumps, and pipes with valves. Ammonia gas leaks are challenging to monitor and detect due to the congestion, as access to some vessels may be blocked.
Therefore, perimeter monitoring is a standard procedure in fertilizer facilities to protect staff and nearby communities.
Ammonia dispersion is affected by the size of the leaks, air temperature, wind, etc.
The equipment and machinery that should be checked, besides the periphery monitoring, are listed below:
- Rails and trucks during loading and unloading
- Shipping port terminals
- Storage tanks
- Spray towers
- Pumps and compressors
- Pipelines and valves
Ammonia Gas Detection
Facility engineers should plan on having a mix of fixed gas detectors for continuous 24/7 monitoring and portable sensors that employees can carry or wear. Several detectors should be installed to ensure the entire factory area is covered.
Special attention must be given to weak points like pipes and valves that can easily corrode. Therefore, these are places where fixed ammonia gas detection must occur. Detection of small leaks can protect employees, allow for repair and preventive measures, and save costs of more extensive repairs and facility shutdowns.
The ammonia sensors nowadays have advanced electronics that can detect trace amounts of ammonia, which is necessary to control exposure to the highly toxic gas.
For example, Interscan’s GasD 8000 Series Portable Gas Analyzers offer three options, which can measure from 0 ppm; two instruments measure up to 50 ppm and 500 ppm, with a resolution of 0.1 ppm. The third option is very sensitive and measures from 0-10 ppm with a resolution of 0.01 ppm. The time needed for measurements is less than one second and happens in real-time, and audio and visual alarms alert users immediately. It has data logging possibilities to keep a record for registering employee safety.
Fertilizer facility engineers must also remember to maintain the detectors and check them regularly with bump testing so employees and employers can rely on the accuracy of the ammonia detection readings.
For more workplace safety practices during ammonia use and leaks, check here.
Safety First
Ammonia production and use is expected to increase in the fertilizer and many other industries. Therefore, it is necessary to understand the risks associated with its use in production facilities. Proper safety monitoring equipment and procedures can go a long way in avoiding accidental injury, death, and capital loss.
Sources
Ammonia. Occupational Safety and Health Administration. (n.d.-b). https://www.osha.gov/chemicaldata/623
Azo Materials. (2018, April 13). Detecting ammonia in fertilizer. AZoM.com. https://www.azom.com/article.aspx?ArticleID=15689
Bose, P. (2023, June 1). Ammonia – A Toxic Gas. Retrieved from https://www.azonano.com/article.aspx?ArticleID=6474
OSHA. (2019, March). 2. What’s Dangerous About Ammonia? https://www.osha.gov/sites/default/files/2019-03/fs2-whatsdangerous.pdf
Padappayil RP, Borger J. Ammonia Toxicity. [Updated 2023 Mar 11]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2023 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK546677/
Right to know hazardous substance fact sheet – the official website. (n.d.-e). https://nj.gov/health/eoh/rtkweb/documents/fs/0084.pdf


