- Methane, propane, butane, hydrogen, acetylene, ethylene, ammonia, silane, and carbon monoxide are the nine gases that are industrial fire hazards.
- Each has specific temperature and concentration ranges in which they pose a fire and explosion risk.
- Continuous air monitoring is necessary to detect the gas before it reaches the minimum flammable levels.
According to the Occupational Safety and Health Administration (OSHA), more than 200 workplace fires occur daily, and injure an average of over 5,000 people annually. Many industries utilize flammable gases, which offer benefits and applications, but pose a significant fire or explosion risk without proper safe handling. It is necessary to understand which gases pose a fire and explosion risk, and what makes them hazardous, to control them effectively. Safety managers and industrial hygienists can learn about flammable gases to keep their workers and facilities safe.
What Causes Fires and Explosions?
Hazardous gases used or generated in industry can be toxic or flammable, posing distinct risks. Some can be both flammable and toxic. Accidents and deaths from flammable gases are fewer than those from poisonous gases, but the damage can be significant.
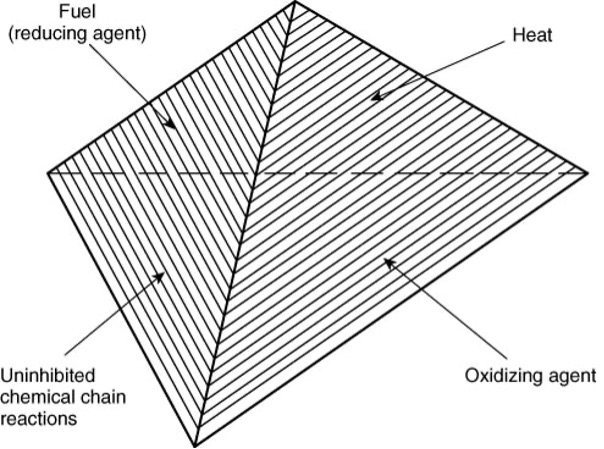
Figure 1: The components necessary for a fire. Fire needs fuel, heat (ignition source), and an oxidizing agent. Sometimes an additional factor is considered- the chance of the chemical reaction driving fires to continue uninhibited, NFPA. (Image credits: https://www.nfpa.org/about-nfpa/press-room/reporters-guide-to-fire/all-about-fire)
Flammable gas risks are fires and explosions. Fires can occur due to electrical sparks, friction, or heat sources. People can be hurt, sometimes fatally, and the facilities can be destroyed. Oxygen and oxidizing gases are not fuels; they do not burn, but they support combustion. Higher concentrations of oxidizers hasten combustion, and materials that do not usually ignite will do so when oxygen levels are above normal.
Fire and explosions are essentially the result of a chemical reaction- oxidation, but that occurs very quickly.
Fire: Three components are necessary for a fire: fuel, oxidizer, and ignition source. The fuel (any flammable material, including gases) reacts with an oxidizer, usually oxygen, when heated or sparked. See Figure 1.
Flash fire: When all three components, fuel, oxidizer, and ignition source, exist, but the fuel is dispersed in the air, a flash fire occurs and can be widespread.
Explosion: In addition to the three elements of fire, an explosion requires a fourth factor. When fuel or gas is dispersed within a built-up space, such as a building or a confined space, the gases expand due to heat from the fire, and pressure builds as they cannot escape, causing an explosion. Explosions can also occur without confinement when the flame spreads rapidly, generating compression waves. In both cases, the damage from explosions is caused by the pressure generated by the rapid release of high energy.
The risks posed by gases depend on the concentrations and the temperatures at which they burn. To control fire risks, safety managers and industrial hygienists must understand these critical parameters.
Flammability
A gas’s flammability or explosive limits are the minimum and maximum concentrations at which it can combust or explode. These limits are percentages of the fuel that exists in the air.
- LEL (Lower Explosive Limit) is the minimum concentration of the gas required for a fire or explosion, even when other factors are present. The LEL is usually less than 5% by volume in the air. The exact percentage varies by gas; for example, the LEL for benzene is 1.2%, but it is 15% for ammonia. If the fuel/gas percentage is below the LEL, the air is too lean to burn.
- UEL (Upper Explosive Limit) is the maximum level of the gas that can burn. If gas or other fuel levels exceed this limit or are too rich, the concentration of oxygen is too low to support a fire.
A gas will cause a fire only if its concentrations are within the flammable range, which is between LEL and UEL, when it comes into contact with an ignition source. However, LEL and UEL indicate average values, and gas does not disperse uniformly in a space. So, there can be air pockets where gas levels exceed LELs. Therefore, when air is monitored for flammable gases, the lowest limit for sounding an alarm can be set at a fraction of the gas LEL, usually 20% to 40% of the LEL, to give people enough time to evacuate the facility and ventilate the area before any danger of a fire arises.
Influence of Temperature
Two critical temperatures must be considered to understand the influence of temperature on the risks posed by gas in producing fires and explosions: flashpoint and auto-ignition temperature; see Figure 2. These temperature definitions are usually used for liquids that vaporize. However, many gases at room temperature can be liquids at very low temperatures, so it is also used for gases.
Flashpoint: The flashpoint is the minimum temperature at atmospheric pressure where a flame can ignite vapors and gases present within their flammable range. It is a crucial parameter that safety managers must consider when storing, transporting, and using flammable gases. Before a gas can burn, its temperature must reach or exceed its flashpoint. At higher pressure, gases will have lower flashpoints or temperatures at which they ignite on contact with a flame.
Autoignition temperature: The temperature at which a flammable gas mixture can ignite without a spark. The range is around 500 to 1000°F and can vary for a gas based on various factors. The surface temperatures should not be allowed to reach these limits to prevent hazards.
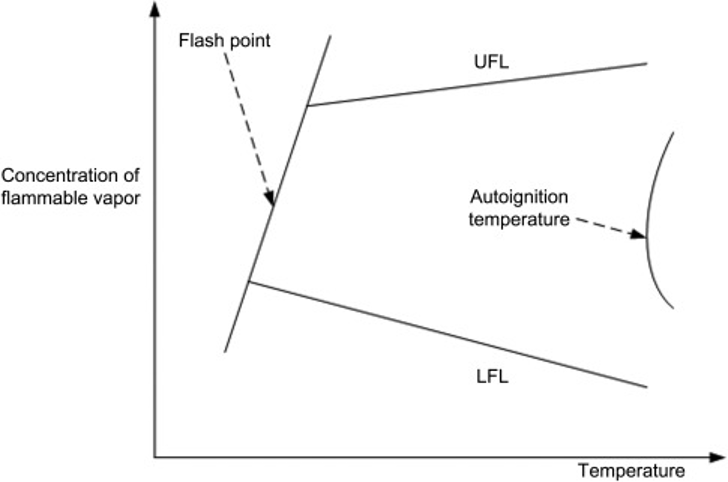
Figure 2: Flammability and ignition limits, Sutton (2017). (Image credits: https://doi.org/10.1016/B978-0-12-812883-1.00001-2)
The boiling point is applicable only to liquids. It is the temperature at which the vapor pressure of a liquid equals the atmospheric pressure. Liquids with low boiling points are volatile.
Based on flashpoint, materials are classified as flammable or combustible. Though these terms are often used as synonyms, the conditions, namely temperature, required for ignition differ.
- Flammable gases are those that are gases at room temperature and have a flashpoint lower than 37.8°C (100°F). Therefore, these gases are more prone to spontaneous combustion because they burn at or near room temperature.
- Combustible gases have a flashpoint at and over 37.8°C (100°F) but below 93.3°C (200°F). Combustible gases have to be heated before they ignite. However, they can also auto-ignite.
A related phenomenon is spontaneous combustion. Materials like hydrocarbons, oily rags, hay, etc., can slowly oxidize in the presence of oxygen in the air, and the reaction can heat the material enough to reach the ignition temperature and start a fire. It differs from auto-ignition, which occurs without outside heat energy as soon as the gas comes into contact with enough oxygen.
Information on the gas’s properties is provided by chemical suppliers in Safety Data Sheets (SDS), as required by OSHA.
Common Flammable Gases and Their Properties
All hazardous gases are flammable because of their flash points. The nine common flammable gases used or found in industry are listed below.
-
Methane (CH4)
The physical properties, according to NOAA, are:
Flash Point: -306°F
LEL: 5 %
UEL: 15 %
Autoignition Temperature: 1004°F”
Methane is a colorless and odorless gas. It is a highly flammable and explosive gas that vaporizes at ambient temperatures. The vapors are lighter than air and may contain explosive compounds when heated. It can also cause asphyxiation.
It is found as a component of natural gas and biogas and is used to make other chemicals.
For more details and information on firefighting, see the NOAA resource.
-
Propane (C3H8)
The physical properties, according to the NOAA, are:
Flash Point: -156°F (gas)
LEL: 2.1 %
UEL: 9.5 %
Autoignition Temperature: 842°F
The gas is colorless with a petroleum-like odor. It is highly flammable and explosive and is heavier than air. The liquid can cause frostbite, and 10% of the gas in the air causes dizziness.
It is transported as liquified gas that is stenched. It is the gas used for cooking, heating, and welding.
For more details and information on firefighting, see this NOAA resource.
-
Butane (C4H10)
According to the NOAA, the physical properties are:
Flash Point: -76°F
LEL: 1.9 %
UEL: 8.5 %
Autoignition Temperature: 550°F
The gas is colorless and has a slight petroleum-like odor. It is highly flammable and explosive. It is also transported as a liquified gas and stenched with a strong scent for leak identification. The liquid form can cause frostbite, and the gas can lead to coughing and shortness of breath.
Butane is used as a fuel in portable heaters and cigarette lighters. It is also used as an aerosol propellant and as isobutane as a refrigerant. Industries use it as a raw material to make chemicals and solvents.
Consult this NOAA resource for more information and methods for firefighting.
-
Hydrogen (H2)
According to the NOAA, the other physical properties are:
LEL: 4 %
UEL: 75 %
Autoignition Temperature: 1065°F
Hydrogen is a colorless and odorless gas that is highly flammable. It is flammable over a wide range of vapor-air mixtures. The gas has the lowest flashpoint of all fuels. The vapors are lighter than air. The gas is not toxic, but it can cause asphyxiation by displacing oxygen.
It is used as a fuel for rockets and welding. Hydrogen is growing in popularity as a clean fuel for vehicles, as it burns to produce water and heat. It is also used to refine petroleum, treat metals, and produce fertilizers.
Consult this NOAA resource for more information and methods for firefighting.
-
Acetylene (C2H2)
According to the NOAA, the other physical properties are:
LEL: 2.5 %
UEL: 100 %
Autoignition Temperature: 581°F
Acetylene is a colorless gas with a faint garlic odor that is lighter than air. It is highly flammable and burns with a sooty flame. The gas can rapidly flashback to the leak source. It can explode after prolonged heating. It can also cause headache, loss of consciousness, and asphyxiation by replacing oxygen.
It is the gas most commonly used for welding, cutting, and soldering metals. It is also used for brazing, in the glass industry, and to produce acetaldehyde and acetic acid.
Consult this NOAA resource for more information and methods for firefighting.
-
Ethylene (C2H4)
According to NOAA, the physical properties are:
Flash Point: -213°F (approx.)
LEL: 2.75 %
UEL: 28.6 %
Autoignition Temperature: 842°F
Ethylene is a colorless gas with a sweet odor that is lighter than air. It is flammable as it ignites readily with a flame. It is explosive after prolonged heating. It can cause drowsiness, unconsciousness, and muscular weakness.
The gas is used in the horticulture industry for fruit ripening and in plastic production.
Consult this NOAA resource for more information and methods for firefighting.
-
Ammonia (NH3)
According to the NOAA, the other physical properties are:
LEL: 16 %
UEL: 25 %
Autoignition Temperature: 1204°F
NH3 is a colorless gas that has a strong odor. It is lighter than air but will initially accumulate at the ground. It is shipped as a liquid. It is usually not flammable; however, at certain concentrations and when in contact with strong ignition sources or other combustible materials, it can be flammable. It is also very corrosive and toxic, which can cause serious and permanent health injury. It is Immediately Dangerous to Life or Health (IDLH) at 300 ppm according to the US National Institute for Occupational Safety and Health (NIOSH).
Ammonia is used as a fertilizer, for indoor refrigeration equipment, and in the manufacture of other chemicals.
Consult this NOAA resource for more information and methods for firefighting.
-
Silane (SiH₄)
Flashpoint, LEL, and UEL are not available.
Boiling point: -169°F
Silane is a colorless gas with a repulsive odor and is lighter than air. It is highly flammable and explosive when heated for prolonged periods. It can spontaneously combust without a heat source. In addition, it is very toxic when inhaled and on skin contact. It can cause dizziness and asphyxiation, especially in confined spaces.
The gas is used to manufacture semiconductors. Because it can ignite spontaneously in the air, caution must be used when handling silane.
Consult this NOAA resource for more information and methods for firefighting.
-
Carbon monoxide (CO)
Flashpoint: Not available
LEL: 12 %
UEL: 75 %
Autoignition Temperature: 1128°F
It is a colorless and odorless gas. It is a flammable gas whose containers can explode if exposed to prolonged heat. It is lighter than air and can flash back rapidly. It is also very toxic and can cause death when inhaled at high concentrations for just an hour.
CO is produced in various industries due to the incomplete combustion of fossil fuels.
Consult this NOAA resource for more information and methods for firefighting.
Fire Risks and Prevention
According to Northwestern University, some of the everyday tasks that are associated with fire and explosion risks are listed below:
Welding, cutting, and brazing: These tasks account for 34% of occupational fires. The work generates flames and sparks, making it a high-risk zone for fires. Inspecting the work area and using a fire watch can minimize this problem.
Electrical equipment: Overloaded or damaged equipment accounts for 22% of workplace fires. Regular checks and maintenance can help overcome these problems.
Accumulation of combustible materials: Any trash, organic material, or paper that accumulates can fuel a fire or hasten its spread. Good housekeeping can remove these materials and keep the pathway to fire extinguishing equipment free.
Storage: Flammable liquids (thinners and aerosols) and gases should be stored properly with sufficient ventilation to prevent leaks and accumulation. Store flammable gases away from oxygen containers. Do not allow smoking around stored gases or at work stations. Some other ways to prevent fire are to reject containers without clear labels.
In addition, verify that valves and caps are closed before handling gas containers. Handle the container carefully without rolling or dropping it,
Areas involving gas storage and use should be monitored continuously with detectors.
Gas Detection Systems
Most of the gases are colorless, and many are odorless. It is not possible to rely on human detection to prevent the dangers posed by gases that are hazardous at low concentrations. Using precise, reliable gas sensors placed at the correct height and location can monitor the air around the clock and alert people when gas levels reach 20-40%, allowing time for corrective action.
Interscan offers AccuSafe, a fixed gas detection system for many flammable gases found in industry.
Find out more about the AccuSafe and how it helps in preventing fires and explosions in your facility.
FAQ
- What are the most common flammable gases found in the workplace?
The most common flammable gases in industrial settings include methane, propane, butane, hydrogen, acetylene, ethylene, ammonia, silane, and carbon monoxide. Each poses unique fire and explosion risks based on their concentration and ignition properties.
2. What is the difference between flammable and combustible gases?
Flammable gases ignite at temperatures below 37.8°C (100°F), making them more prone to fire at room temperature. Combustible gases have higher flashpoints (up to 93.3°C or 200°F) and require more heat to ignite but can still auto-ignite under certain conditions.
3. How can flammable gas fires and explosions be prevented in the workplace?
Prevention includes continuous gas monitoring, proper storage, routine equipment checks, housekeeping, and employee training. Alarm systems should trigger when gas levels reach 20–40% of the Lower Explosive Limit (LEL) to allow early evacuation and ventilation.
Sources
Chrisholm, K. (2022, Jan 20). Flammability vs. Combustibility — What’s the Difference? Retrieved from https://www.hazmatschool.com/blog/flammability-vs-combustibility-whats-the-difference/#:~:text=The%20term%20combustible%20can%20also,higher%20flashpoint%20than%20flammable%20materials.
Kuhl, A., & Reichenbach, H. (2008). Combustion effects in confined explosions. Proceedings of the Combustion Institute, 32(2), 2291-2298. https://doi.org/10.1016/j.proci.2008.05.001
NFPA. (n.d.). Reporters Guide: All About Fire. Retrieved from https://www.nfpa.org/about-nfpa/press-room/reporters-guide-to-fire/all-about-fire
NOAA. (2013, Aug 13). Flammable Levels of Concern. Retrieved from https://response.restoration.noaa.gov/oil-and-chemical-spills/chemical-spills/resources/flammable-levels-concern.html
Northwestern University. (2024, Feb). Fire Prevention. Retrieved from
https://www.northwestern.edu/environmental-health-safety/training/newsletters/volume-8-issue-2-february-2024.html#:~:text=The%20Occupational%20Safety%20and%20Health,injured %20by%20workplace%20fires%20annually.
O’Connor, B. (2023, Mar 27). Explosions, Deflagrations, and Detonations. Retrieved from
Santos, S. M., Nascimento, D. C., Costa, M. C., Neto, A. M., & Fregolente, L. V. (2020). Flash point prediction: Reviewing empirical models for hydrocarbons, petroleum fraction, biodiesel, and blends. Fuel, 263, 116375. https://doi.org/10.1016/j.fuel.2019.116375
Sutton, I. (2017). Chapter 1 – Safety in Design. In Plant Design and Operations (Second Edition). Elsevier Inc, 1-34. https://doi.org/10.1016/B978-0-12-812883-1.00001-2
University of Pittsburgh Safety Manual. (2020, Aug 6). Storage and handling of flammable and combustible liquids. Retrieved from https://www.safety.pitt.edu/sites/default/files/02-003FlammableLiquid.pdf


