- Gases that can pose occupational hazards can be generated during wastewater treatment processes or used to decompose organic matter and disinfect.
- Methane, hydrogen sulfide, ammonia, carbon dioxide, carbon monoxide, phosphine, and nitrous oxide are produced in wastewater plants and sewage systems.
- The hazardous gases used for wastewater treatment are chlorine, chlorine dioxide, ozone, and sulfur dioxide.
- Monitoring the air continuously in high-risk work zones and using portable gas detectors in confined spaces is essential for limiting the occupational hazards posed by these gases.
Wastewater treatment is a significant industry that is expected to grow at a 6.87% CAGR between 2025 and 2030. As fresh water resources become scarce and industrial pollution increases, countries worldwide are focusing on treating wastewater for environmental protection and reuse. Wastewater centers are being built in rural and urban areas, and more people are being employed in the industry. Therefore, the occupational hazards the staff faces are also receiving more attention. In this article, industrial hygienists and safety managers can learn about the hazardous gases encountered in this sector.
Hazardous Gases in Wastewater Treatment
Wastewater is treated to remove suspended particulates, organic matter, pathogens, industrial waste, toxic chemical pollutants, and fertilizers (nitrates and phosphorus). The sewers are one of the confined spaces that transport wastewater to plants, where workers must enter for specific tasks. Wastewater treatment involves three stages: primary, secondary, and tertiary, in which mechanical, biological, and chemical methods are used, respectively, to purify the water.
- Mechanical treatment is the first step and removes coarse solids, paper, food waste, grit, grease, sand, and gravel.
- Biological treatment is the second step, in which microorganisms, including bacteria, decompose the organic matter in wastewater. The processes can be aerobic (in the presence of oxygen) or anaerobic (in the absence of oxygen). Several hazardous gases are formed at this stage.
- Chemical treatment is the last stage, in which plant nutrients such as phosphorus and arsenic are removed by chemical addition.
After impurities are removed, water is disinfected before discharge.
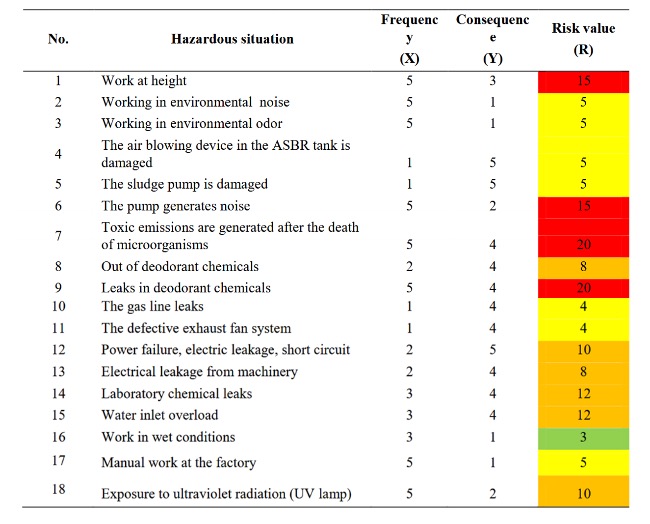
TABLE 1. “The level of risk in the Di wastewater treatment plant.” (Red: extreme risk; orange: high risk; yellow: medium risk; green: low risk) Tron & Than (2020). (Credits: https://doi.org/10.37550/tdmu.EJS/2021.01.154
The gases that workers encounter in wastewater treatment plants and sewage systems fall into the following two categories:
Generated gases: Some gases are produced during the decomposition of organic matter and other waste in plants and sewage systems, and they can accumulate to dangerous levels, especially in confined spaces like sewers or tanks.
Treatment gases: Many gases used for wastewater treatment can be hazardous.
Emissions are among the top risks workers face in treatment plants and sewers. For example, in a Vietnamese wastewater treatment plant, workers are at extreme risk from emissions, high risk from laboratory chemical leaks, and medium risk from tank and gas line leaks, as shown in Table 1.
Hazardous gases can be toxic, corrosive, flammable, and explosive, and safety measures and equipment must be customized for the emissions in each area. Several of the gases are also greenhouse gases that cause climate change.
Sewer Gases
The so-called “sewer gases”, mainly produced by wastewater decomposition, that are hazardous to workers include methane, hydrogen sulfide, ammonia, carbon dioxide, carbon monoxide, phosphine, and nitrous oxide.
- Methane
Methane (CH4) is produced in large amounts from the sludge in anaerobic digesters. Domestic and industrial waste is a source of CH4, which is also generated in sewers. Most waste treatment plants use aerobic processes, but when large amounts of solids are present, CH4 can form. Some of it escapes into the air as fugitive emissions. Methane is the main volatile organic compound in wastewater treatment systems.
Methane is a colorless and odorless gas.
- It is hazardous because it is flammable in concentrations of 5-15% of the atmosphere.
- It is also a physical asphyxiant, i.e., one that can displace oxygen (O2), producing oxygen-deficient (hypoxic) conditions in confined spaces. Initial symptoms can be headache, tachycardia at low levels of CH4, but as its levels rise and O2 levels fall, it can be fatal.
- Methane is also a greenhouse gas with a high global warming potential (GWP).
Find out how to control CH4 effects using this resource.
-
Hydrogen Sulfide
Hydrogen Sulfide (H2S) is generated when organic matter with sulfur is decomposed. In sewage, the anaerobic breakdown of animal and plant proteins by bacteria also produces H2S.
H2S is a colorless gas with a distinct rotten-egg odor. However, at high concentrations above 150 ppb, where it poses a health hazard, it numbs the olfactory senses. Hence, people are unable to smell it, leading to a false sense of security. H2S is flammable, toxic, corrosive, and a chemical asphyxiant.
- It is toxic when it is inhaled as a gas and irritates the respiratory tract and eyes. It is fatal at concentrations of 100 ppm.
- Being corrosive, it can damage pipelines, equipment, and infrastructure and cause partial or total loss of function.
- As a chemical asphyxiant, H2S interferes with O2 delivery within the human body, causing dizziness, headache at low levels, and eventually death.
- It is also flammable and a greenhouse gas.
H2S is considered the most concerning gas and the primary source of malodor in sewers. This resource lists its safety measures.
-
Ammonia
Ammonia (NH3) has two sources in the wastewater systems: (1) runoff from streets, lawns, and farms containing ammonia enters the wastewater system; (2) the decomposition of animal and human waste also produces NH3.
Ammonia is a gas at room temperature with an intense, distinctive odor. NH3 is toxic, corrosive, flammable, and a greenhouse gas.
- Inhaling NH3 can cause respiratory, eye, and skin irritation following acute and chronic exposure. High concentrations can cause lung damage.
- Ammonia is corrosive and damages certain metals.
- NH3 is flammable and explosive at concentrations of 15-28% in air.
This resource provides details on controlling NH3.
-
Carbon Dioxide
Carbon dioxide (CO2) is found in wastewater systems for four reasons.
- As a major atmospheric gas, it can dissolve in wastewater.
- Aerobic microbes used in biological treatments also produce CO2 during respiration.
- CO2 is produced when carbonates and bicarbonates in wastewater react with acids.
- CO2 is produced during anaerobic decomposition of organic matter, forming biogas, a mixture of methane (60-70%) and CO2 (30-40%).
It can also be added to adjust pH levels in wastewater, as it dissolves in water to form carbonic acid.
CO2 is a colorless and odorless gas at room temperature. It is not toxic or flammable; however, it is a physical asphyxiant that can produce hypoxic conditions with severe health problems and eventual death at high concentrations of CO2. People should be careful about CO2 accumulation in confined spaces, where levels above 1% can be dangerous. CO2 is also a greenhouse gas.
-
Carbon Monoxide
Carbon monoxide is produced biotically and abiotically and is an occupational hazard for workers.
- Biotically, it is produced in aerobic digestors from the decomposition of animal organic waste by acetogenic, methanogenic, or sulfate-reducing bacteria.
- Abiotic formation occurs due to high temperatures, visible or UV radiation, and humidity. Thermal degradation of matter at temperatures below 100 °C can also produce CO.
Carbon monoxide is a colorless, odorless gas that is toxic, flammable, and a chemical asphyxiant.
It is a health hazard as it causes sore muscles, irritability, nausea, irregular heartbeats, and death in high concentrations. Find out the permissible levels and safety measures in this NJ Health resource.
-
Nitrous Oxide
Nitrous oxide (N2O) is produced during biological nitrogen removal via nitrification and denitrification. It is emitted in three main areas in a wastewater treatment plant-
- The primary source (90%) of the N2O emission is the activated sludge compartments.
- The other two sources are grit tanks (5%) and sludge storage tanks (5%).
Nitrous oxide is a colorless gas with a sweet odor. It is hazardous because of its toxicity.
- N2O is toxic when inhaled and when it comes into contact with the skin and eyes. It can have acute and chronic health problems of the lungs, kidneys, and nervous system. It causes mutations and is therefore a reproductive hazard.
- Though N2O is not flammable, it can increase the intensity of a fire because it is an oxidizer. It can help ignite paper, wood, and oil.
N2O is also a potent greenhouse gas. Find out the permissible limits and safety measures for N20 in this NJ Health resource.
-
Phosphine
Phosphine (PH3) is generated during biological anaerobic processes by microbes from phosphides and phosphates.
PH3 is a colorless gas with a fishy or garlic-like odor. In addition to H2S, PH3 is responsible for the foul smell in wastewater systems.
PH3 is flammable, explosive, corrosive, and toxic.
- Chronic exposure to the gas can be detrimental to health. During acute exposures, concentrations of 500 ppm are lethal in 30 minutes, and 1,000 ppm of NH3 is lethal after a few breaths.
- Phosphine is highly flammable and explosive. It is heavier than air, so it accumulates at the bottom and travels to an ignition source.
- PH3 is very reactive and, when pure, can ignite spontaneously in air.
Find out safety measures and more information for PH3 in this resource.
Treatment Chemicals
To prevent the spread of waterborne pathogens, wastewater is treated with disinfectants before it is released into the environment or reused. In addition, chemicals are also used in the tertiary stage. Many of these are hazardous and pose occupational risks due to accidents during transport, shipping, and storage, or to leaks from equipment. These gases are chlorine, chlorine dioxide, ozone, and sulfur dioxide.
-
Chlorine (Cl2)
Chlorine (Cl₂) is commonly used for disinfection because it kills or deactivates pathogens (bacteria and viruses), dissolves in water, is stable in storage, and is affordable. It is added as a gas or sodium hypochlorite.
Chlorine is a greenish-yellow gas and has a bleach-like odor. It is hazardous because it is toxic and a strong oxidizer.
- Cl2 has detrimental health effects from acute to chronic exposure. High concentrations can cause pulmonary edema in acute exposure. Chronic exposure can damage the lungs and teeth.
- As an oxidizer, it reacts with several combustible compounds, thereby increasing the risk of fire and explosions.
-
Chlorine Dioxide
Chlorine dioxide (ClO2) is used for disinfection to deactivate viruses and other microbes resistant to chlorine and remove algae. However, it forms and leaves significant amounts of chlorite and chlorate in the water, which can pose a health risk to people who drink it. It breaks down to Cl2 and O2 in air.
ClO2 is a yellow-to red gas with a bleach-like odor similar to Cl2. It is hazardous because it is toxic, flammable, and reactive. ClO2 can irritate the respiratory system, eyes, and skin after acute and chronic exposures. Chronic effects last for years.
Please find out more about the gas and safety measures to control its effects in this resource.
-
Ozone
Ozone (O3) is more effective than chlorine in destroying viruses, protozoa, and bacteria. It is a strong oxidant that attacks microbes. It also removes iron, manganese, and arsenic through oxidation. It is also used during the secondary and tertiary stages to improve the biodegradability of organic matter and to remove pollutants, respectively. It is expensive and was not widely used. Still, since it can act alone or in combination with other oxidizers to remove organic matter, it is becoming more popular for increasing water recycling and reuse.
Ozone is a colorless gas and has a pungent odor. It is hazardous because it is toxic, corrosive, and a strong oxidizer.
- O3 is poisonous and poses a health risk following acute and chronic exposure. It can irritate the lungs, eyes, and skin, and, in higher acute exposures, can cause edema. Chronic exposures can lead to lung damage, cancer, and mutations, making it a reproductive hazard.
- O3 is corrosive and acts on many metals. Stainless steel must be used for handling ozone.
Use this resource for more details and safety measures to protect workers from ozone.
-
Sulfur Dioxide
Sulfur dioxide (SO2) is used to remove chlorine disinfectant residues from water. It lowers pH when dissolved in water, thereby improving arsenic removal from acid wastewater.
SO2 is a colorless gas with a pungent smell. It is hazardous because it is toxic. At higher concentrations, it causes edema following acute exposure. Chronic exposure can cause bronchitis, loss of smell, headaches, dizziness, and nausea.
Use this resource for more details and safety measures for SO2.
Monitoring Hazardous Gases
Based on the hazards posed by the gases, safety managers and industrial hygienists must select appropriate protective measures for workers. However, in every case, areas with a high risk of gas emissions must be monitored to ensure gas concentrations remain within permissible levels. It is essential to use fixed sensors in high-risk workplaces for continuous monitoring and to use portable gas analyzers before entering confined spaces.
Interscan offers AccuSafe for fixed-point gas detection and the GasD® 8000 Portable Gas Monitor for over 20 gases. The sensors Interscan offers detect most hazardous gases found in wastewater systems.
Schedule a consultation with our experts at Interscan to find out more about our gas detection systems.
Sources
ATSDR. (2011, Nov 29). Public Health Statement for Chlorine Dioxide and Chlorite. Retrieved from https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=580&toxid=108
Caniani, D., Esposito, G., Gori, R., & Mannina, G. (2015). Towards A New Decision Support System for Design, Management and Operation of Wastewater Treatment Plants for the Reduction of Greenhouse Gases Emission. Water, 7(10), 5599-5616. https://doi.org/10.3390/w7105599
Chou, M. S., & Cheng, W. H. (2005). Gaseous emissions and control in wastewater treatment plants. Environmental engineering science, 22(5), 591-600.
EPA. (199). Wastewater Technology Fact Sheet- Ozone Disinfection. Retrieved from https://www.epa.gov/sites/default/files/2015-06/documents/ozon.pdf
International Labour Organization. (2022, July 30). Hazards in Sewage (Waste) Treatment Plants. Retrieved from https://www.iloencyclopaedia.org/part-xvii-65263/public-and-government-services/item/831-hazards-in-sewage-waste-treatment-plants
Paramerta, A., Nadhifah, D.F., Marques, M.G.J.V., et al. (2024). Chlorine residuals in wastewater treatment: implications for pathogen inactivation and environmental impact. JURNAL PENELITIAN DAN KARYA ILMIAH LEMBAGA PENELITIAN UNIVERSITAS TRISAKTI, 9 341-347.Doi: https://doi.org/10.25105/pdk.v9i2.18987
Nair, A., Jamshed, N., & Kumar, G. (2025). Sewer Gas Toxicity: A Literature Review.
Indian Journal of Forensic Medicine and Toxicology, 19 (3). DOI:10.37506/m15nmz02
Namour P. (2022). The biogeochemical origin of sewage gases and control of their generation. Journal of Hazardous Materials Advances. 7:100124.
Pereira, C. P., Goldenstein, J. P. N., & Bassin, J. P. (2022). Industrial wastewater contaminants and their hazardous impacts. Biosorption for wastewater contaminants, 1-22.
Sayers, R. R. (1934). Gas hazards in sewers and sewage-treatment plants. Public Health Reports (1896-1970), 145-155.
Stegenta-Dąbrowska, S., Drabczyński, G., Sobieraj, K., Koziel, J. A., & Białowiec, A. (2019). The biotic and abiotic carbon monoxide formation during aerobic co-digestion of dairy cattle manure with green waste and sawdust. Frontiers in Bioengineering and Biotechnology, 7, 283.
Tron, H. T., & Than, N. H. (2021). Hazard identification and risk assessment in wastewater treatment plant of Di An City. Thu Dau Mot University Journal of Science, 3(1), 15. https://doi.org/10.37550/tdmu.EJS/2021.01.154
Xu, M. Y., Lin, Y. L., Zhang, T. Y., Hu, C. Y., Tang, Y. L., Deng, J., & Xu, B. (2022). Chlorine dioxide-based oxidation processes for water purification: A review. Journal of Hazardous Materials, 436, 129195.


