- Hydrogen cyanide gas and liquid are poisonous and cause instantaneous death at high concentrations.
- Short-term acute and high exposures can leave people with severe disabilities.
- Using precision detectors to measure the hydrogen cyanide levels is one of the few measures nylon manufacturers must use to protect their staff.
Hydrogen cyanide is a practical industrial reagent used to make engineering nylon. However, the industry suffers some of the highest fatalities caused by this gas in any sector. Therefore, facility safety supervisors should acquaint themselves with the dangers of the compound and how they can prevent them through hydrogen cyanide detection.
Hydrogen Cyanide Use in Nylon Industry
Hydrogen cyanide (HCN) is a toxic gas and liquid which is an acute poison. It is a highly volatile, colorless gas with an almond-like odor. It is light blue as a liquid, boiling at 25.6 °C, making it flammable.
Hydrogen cyanide is a well-known poison used by ancient Egyptians, Romans, and recently in Germany during World War II.
Hydrogen cyanide occurs naturally in apricots, peach pits, and bitter almonds. Adults must consume many apricot pits (50-70) to suffer fatality; for children, it is only 7-10 pits.
Several industries use hydrogen cyanide, such as pulp, textiles, and plastics production, electroplating, fumigating buildings, ships, orchards, and foods. One of the leading global applications of hydrogen cyanide is to produce adiponitrile. Around 92 percent of adiponitrile makes nylon 6,6 resins and fibers.
Nylon 6,6 is a strong and flexible engineering thermoplastic with broad applications in the automotive, electronics, and textile industries. The increase in the growth of light automotive manufacturing generates a higher demand for hydrogen cyanide to produce nylon.
Yet, hydrogen cyanide’s high toxicity has hampered more use even though it is a useful industrial raw material. Many deaths have been recorded in the units manufacturing acrylonitrile–styrene copolymers, so improving safety measures in this industry is vital.
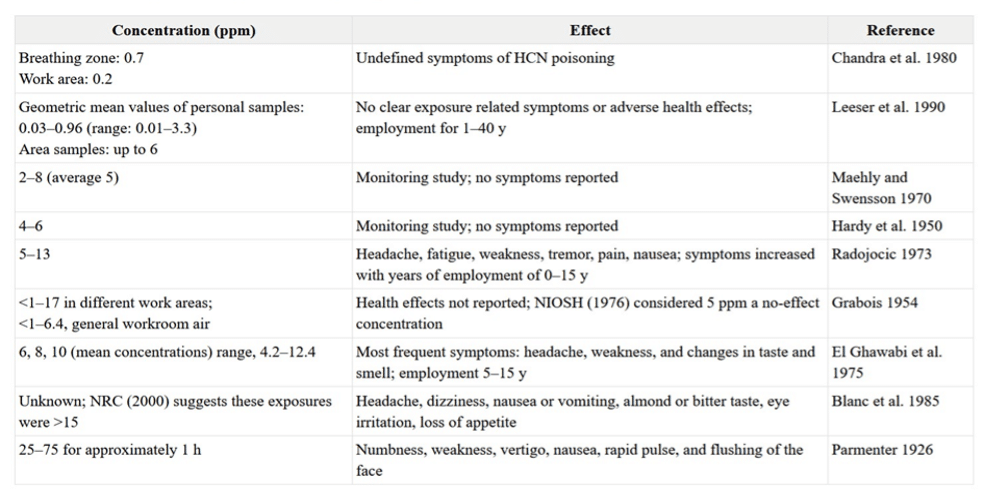
Table 1: Hazards due to occupational exposure to hydrogen cyanide. (Credits: https://www.ncbi.nlm.nih.gov/books/NBK207601/table/ttt00077/)
Hydrogen Cyanide’s Toxicity
Hydrogen cyanide can be fatal in exposure to workers by all routes- inhalation, oral, or dermal contact. The work, duration, and dosage affect exposure impact. Hydrogen cyanide is absorbed and distributed rapidly within the body after exposure and affects oxygen use, causing hypoxia that harms the brain, heart, lungs, and blood vessels. Therefore, symptoms develop immediately. Hydrogen cyanide also leads to metabolic acidosis.
Acute Exposure Effects
Lower dose exposure over five ppm can cause hypoxia symptoms like flushing, dizziness, light-headedness, and headache, see Table 1. Dermal exposure causes rash and dermatitis.
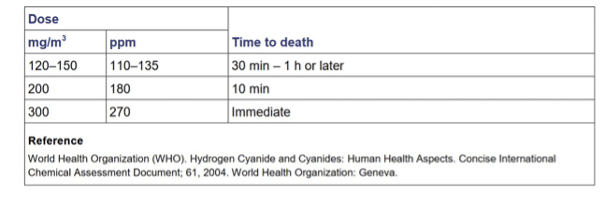
Early symptoms of severe exposure include nausea, faintness, headache, confusion, weakness, convulsions, tachycardia, and tachypnea. Later developments are seizures, deep coma, pulmonary edema, respiratory depression and arrest, cardiovascular collapse, and death. Inhaling very high doses can make people unconscious and cause coma and death within seconds; see Table 2 for the amount and duration that can lead to fatalities. Ingestion and dermal contact can lead to death within minutes. The lethal oral dose of hydrogen cyanide is 50-90 mg.
Brief single exposure to low levels of hydrogen cyanide has no long-term effects.
However, a larger exposure can cause long-term CNS damage, intellectual deterioration, memory deficits, personality changes, and Parkinsonism.
Chronic Exposure Health Effects
Long-term repeated exposure to low hydrogen cyanide causes neurological symptoms and affects the optic neuropathy and thyroid, see Table 1.
Hydrogen cyanide is not carcinogenic or mutagenic. Cyanide doesn’t accumulate in the body as it is converted to thiocyanate, quickly and easily excreted through urine.
Table 2: “Time to death following hydrogen cyanide inhalation by humans.” (Credits: Public Health England )
Environmental Effects
Hydrogen cyanide has a half-life of 1-3 years in the air and volatilizes from water, where its half-life is many hours to days. It is highly soluble in water and very toxic, affecting aquatic life, and has long-lasting effects. The compound doesn’t enter and pollute soils.
Gas and liquid hydrogen cyanide are flammable at 26oC, around the ambient temperature, and the compound is a fire hazard at concentrations of 5.6% (v/v). Any spark or flame can be dangerous and lead to explosions.
Hydrogen Cyanide Deaths
Many accidental exposures to hydrogen cyanide at work are of low dosage and have little or no adverse effects; however, many deaths also occur. According to the ‘American Association of Poison Control Centres’ intentional poisonings accounted for only 10 percent of hydrogen cyanide deaths in 2014, so the rest and most deaths happen as occupational hazards.
Hazardous air pollutant (HAP) emissions of hydrogen cyanide occur from storage vessels and equipment leaks. Another source of emissions is accidents during transportation and storage.
Workplace Exposure Limits
Due to the toxic nature of gaseous and liquid hydrogen cyanide, there are strict limits that manufacturers using the material must observe to protect their workers. The USA and the UK have set the airborne limit at ten ppm.
In the USA, the following limits apply for airborne exposure:
- Occupational Safety and Health Act (OSHA) airborne permissible exposure limit (PEL) over an 8-hour work shift that is legally applicable is an average of 10 ppm.
- The National Institute for Occupational Safety & Health (NIOSH) recommends that an airborne exposure limit (REL) of 4.7 ppm not be exceeded at any time.
- The American Conference of Governmental Industrial Hygienists (ACGIH) recommends a threshold limit value (TLV) of 4.7 ppm not be exceeded at any time.
If people have dermal contact with hydrogen cyanide, even the concentrations lower than the above limits can be too much and lead to overexposure. Therefore, it explains why hydrogen cyanide detection is critical.
The Need for Hydrogen Cyanide Detection
Though hydrogen cyanide has a characteristic odor that can be smelt above 1 ppm, not everyone can smell it. Genetics controls the ability to smell hydrogen cyanide, and 25 percent of the population can’t smell the compound.
Therefore, odor is not a safe indicator of the compound.
A more reliable indicator is sensors that can detect and measure even trace amounts of the gas in real time. Using a mix of fixed and portable sensors will provide better protection to workers. Fixed sensors should be installed at identified emission points, and people can carry portable devices, too.
The efficiency of detectors is determined by the lower sensing limit, which indicates the least amounts of toxins the instrument can measure.
For example, Interscan produces three devices in the GasD 8000 Series Portable Gas Analyzers that measure hydrogen cyanide. The lower measuring limit for the three is 0 ppm. The upper limits are 2000 ppb, 20 ppm, and 200 ppm. So, all three are efficient and valuable for hydrogen cyanide detection.
Additional Safety Measures
In addition to detection, the manufacturers must provide protective face and complete body equipment and clothing. Several recommended practices must be followed, like covering areas with dangerous chemical reactions or providing ventilation. The workers must also be informed of the hazards and trained in safety procedures. More information on prevention and safety measures that manufacturers can follow to keep their staff safe can be found in this document. Reducing injuries and deaths in manufacturing units will allow more applications of this valuable chemical.
 Vijayalaxmi Kinhal
Vijayalaxmi Kinhal
Science Writer, CID Bio-Science
Ph.D. Ecology and Environmental Science, B.Sc Agriculture
Sources
Bocos-Bintintan, V., & Ratiu, I.A. (2021). Fast Sensing of Hydrogen Cyanide (HCN) Vapors Using a Hand-Held Ion Mobility Spectrometer with Nonradioactive Ionization Source. Sensors, 21, 5045. https://doi.org/10.3390/s21155045
Centers for Disease Control and Prevention. (2019, June 21). Hydrogen cyanide. Centers for Disease Control and Prevention. https://www.cdc.gov/niosh/topics/hydrogen-cyanide/default.html
Chump, E. L. (2000, May). Economic Impact Analysis For the Proposed Cyanide
Manufacturing NESHAP. Retrieved from https://www3.epa.gov/ttnecas1/regdata/EIAs/Cyanideeia.pdf
Gidlow, D., (2017). Hydrogen cyanide—an update. Occupational Medicine, 67(9), 662–663, https://doi.org/10.1093/occmed/kqx121
Hydrogen cyanide market size & share analysis – industry research. (n.d.-a). https://www.mordorintelligence.com/industry-reports/hydrogen-cyanide-market
National Research Council (US) Subcommittee on Acute Exposure Guideline Levels. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 2. Washington (DC): National Academies Press (US); 2002. 5, Hydrogen Cyanide: Acute Exposure Guideline Levels. Available from: https://www.ncbi.nlm.nih.gov/books/NBK207601/
New Jersey Department of Health. (2011). Right to know hazardous substance fact sheet – the official website. Retrieved from https://nj.gov/health/eoh/rtkweb/documents/fs/1013.pdf
Public Health England. (2016, February). Hydrogen Cyanide Toxicological Overview. Retrieved from https://assets.publishing.service.gov.uk/government/uploads/system/ uploads/attachment_data/file/500821/Hydrogen_Cyanide_PHE_TO_120216.pdf
ECHA. (n.d.). Substance information. Retrieved from https://www.echa.europa.eu/web/guest/substance-information/-/substanceinfo/100.000.747


