- Electrochemical sensors react chemically with target gases and measure the current produced for quantitative estimation.
- The electrochemical sensors are useful for several toxic, corrosive, explosive, and environmentally polluting gases.
- The electrochemical sensors have become state-of-the-art technology due to their accuracy, robustness, size, and cost for industrial use.
Gas sensors are necessary to monitor toxic and hazardous gases in industries to keep their staff, facility, local communities, and the environment safe. Electrochemical gas sensors can detect and quantitatively measure several common gases encountered across industrial sectors. Material science advances have improved the process, and miniaturization has extended its use onsite for real-time monitoring and registering changes in gas concentrations. In this article, you will learn how electrochemical gas sensors work.
Electrochemical Gas Sensors
Several solid-state gas-sensitive gas detectors exist, such as acoustic, catalytic, semiconductor, electrochemical, optical, and thermoelectric sensors.
Electrochemical gas sensors’ react with target gas concentration through oxidation and reduction and measure the resultant electric current, which is proportional to the gas concentrations. Electrochemical sensors are not as accurate as UV-vis spectroscopic instruments. Still, their versatility, compactness, robustness, and cost have made them state-of-the-art gas sensors relied on by many industries for gas detection. They can also be an alternative to traditional, more expensive devices using gas chromatography and infrared spectroscopy.
Kohlraush and Haber developed the first electrochemical cells in 1885 and in the early 1900s. The initial design has undergone several improvements and changes. This optimization process is ongoing, and continuous R&D efforts are directed at improving the instruments’ performance by focusing on response time, sensitivity, gas concentration range, operating temperature, and thermodynamic stability.
The Hardware
The crucial elements of the electrochemical sensors necessary for its function are electrodes, electrolytes, a membrane, and a counter housed in a plastic container.
Electrodes: The sensors can have two to four electrodes immersed in an electrolyte. The electrodes are made of a noble metal like gold or platinum and have a high surface area for faster reactions.
- The working/sensing electrodes (or anode) are fixed to the membrane exposed to ambient air and are dipped in electrolytes. The electrical current enters the system through the anode reacting with the target gas.
- In two electrode configurations, the counter electrode (or cathode) is the point from where the current leaves the sensor.
- The reference electrode is the electrode from which current output is measured in a three-electrode configuration.
Gas permeable membrane: At the input, a porous, hydrophobic membrane in contact with the working electrode controls the gas flow rate into the device, filters unwanted particles, and prevents the electrolyte from leaking out.
Electrolyte: The liquid usually used as the electrolyte is acid, but it can also be an organic compound, depending on the gas that must be detected. The cell reaction occurs in the electrolyte, and the liquid is the medium through which ionic charge/current passes between the anode and cathode.
An exit hole for the gas and current occurs at one end of the device, as shown in Figure 1.
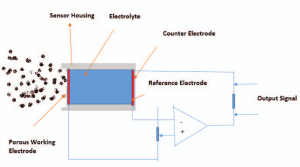
Figure 1: Schematic diagram of electrochemical gas sensor, Yadav et al. (2015). (Image credits: DOI:10.1109/ISPTS.2015.7220127
The Operating Principle
The ambient air diffuses into the sensor through the hydrophobic membrane to reach the working electrode attached, which is reduced or oxidized depending on the target gas. If the electrochemical reaction is oxidation, electrons are released, or the current flows through the electrolyte from the working electrode to the counter electrode. During reduction, the current flows opposite from the counter to the working electrode. The current generated is proportional to the gas concentration in either case.
The current is amplified and reads as a percentage volume or parts per million (PPM). When no target gas is detected, the reading is zero. Electrochemical sensors require a zero or balance adjustment.
Variations Exist
Sensors can differ in size, number of electrodes, and materials used to produce the components depending on the target gas to be measured. The end design compromises sensor performance parameters such as response time, selectivity, sensitivity, and operating life.
For example, a gas sensor for measuring low concentrations of a gas must have high sensitivity. To facilitate this, the hydrophobic membrane will have coarse porosity so that more gas molecules reach the working electrode through the large pores. However, the electrolyte loss due to evaporation through the large pores is higher, reducing sensor operational life.
Dealing with Cross-Sensitivity
The choice of electrolytes and electrode material depends on the reactivity of the target gas to provide better selectivity, but it can reduce sensitivity. Electors can be coated with films and modified with mediators or enzymes to increase selectivity.
Despite being designed for a specific gas, sensors can suffer from cross-sensitivity, where the tools will respond to other non-target gases. Cross-sensitivity occurs when the non-target gases have higher chemical reactivity than the target gas. Non-target gases can sometimes mask target gas, so filters are crucial. A low and specific bias voltage also helps to reduce inaccuracies due to cross-sensitivity.
Besides the components, various external factors can influence the electrochemical sensor’s performance parameters.
Factors Affecting Sensor Accuracy
Temperature and humidity are critical external factors affecting sensor accuracy for various reasons.
Chemical reaction rates are proportional to temperature, so variations in ambient temperature can influence sensor accuracy. Many industrial workplaces have a broader temperature range than usual. So, tools need temperature compensation to maintain accuracy.
Humidity variations cause high-amplitude current fluctuations, resulting in capacitance fluctuations. Ambient humidity and temperature variations will also change acid concentrations in porous electrodes to cause capacitance fluctuations and baseline changes. Baseline currents rely on slight differences between the potentials of working and counter electrodes due to a gradient of acid concentrations. Even slight temperature and acid concentration changes can impact reactions determined by potentials. Hardware changes are needed to correct these errors.
Electrochemical Sensor Applications
Electrochemical sensors have several applications, especially in industries, for ensuring safe working conditions and timely detection of toxic gases injurious to personnel health and explosive gases that harm people and facilities. The various industrial gases that electrochemical sensors can monitor include:
- Environmental pollutants like carbon dioxide, carbon monoxide, nitrogen oxides, sulfur oxides, volatile organic compounds, hydrogen fluoride, formaldehyde, and hydrogen sulfide.
- Explosive and combustive gases like methane, acetylene, ammonia, chlorine dioxide, and formaldehyde.
- Corrosive gases like chlorine, ammonia, hydrogen chloride, sulfur dioxide, and nitrogen dioxide.
- Oxygen is monitored to ensure it does not fall below safe levels in some industries, medical facilities, and food quality control.
Choosing Reliable Suppliers
To get the most benefit from electrochemical sensors, choosing a reliable supplier who provides adequate support and services is essential to ensuring safe working conditions in any industrial facility. Interscan offers accurate and reliable electrochemical devices for detecting over 20 hazardous gases. The company offers fixed and portable sensors suitable for various industries and situations.
Find out more about Interscan electrochemical sensors.
Source
Ali, F.A., Mishra, D.K., Nayak, R. et al. (2022). Solid-state gas sensors: sensing mechanisms and materials. Bull Mater Sci 45, 15. https://doi.org/10.1007/s12034-021-02581-5
Awang, Z. (2014). Gas sensors: A review. Sens. Transducers, 168(4), 61-75.
Bhoga, S. S., & Singh, K. (2007). Electrochemical solid state gas sensors: An overview. Ionics, 13, 417-427.
Farquhar, A. K., Henshaw, G. S., & Williams, D. E. (2021). Understanding and correcting unwanted influences on the signal from electrochemical gas sensors. ACS sensors, 6(3), 1295-1304.
Lagutin, A.S., Vasil’ev, A.A. (2022). Solid-State Gas Sensors. J Anal Chem 77, 131–144. https://doi.org/10.1134/S1061934822020083
Science Direct. (n.d.). Electrochemical Sensor. Retrieved from
https://www.sciencedirect.com/topics/engineering/electrochemical-sensor
Yadav, U., Sarje, R., Shaligram, A. D., & Gangal S. A. (2015). Design, simulation, fabrication and testing of electrochemical NO2 gas sensor. 2nd International Symposium on Physics and Technology of Sensors (ISPTS), Pune, India, 268-272, doi: 10.1109/ISPTS.2015.7220127.