- Peracetic acid’s strong oxidant properties make it an excellent biocidal useful for decontamination, disinfection, and sterilization.
- It is highly reactive and corrosive, making it an explosion risk and resulting in various health issues.
- People involved in its production and use must be adequately protected in the pharmaceutical industry.
Peracetic acid is a valuable and sustainable chemical used for decontaminating, disinfection, and sterilizing in the pharmaceutical industry. However, it is classified as a special health hazard substance and needs to be monitored regularly to avoid exposure. Safety managers should acquaint themselves with this chemical’s effects and how to deal with it, as it is essential to prioritize safety and minimize exposure to peracetic acid in the pharmaceutical industry.
Peracetic Acid Use in the Pharmaceutical Industry
Peracetic acid is a colorless liquid and has a strong vinegar-like odor. It is formed through a reaction of hydrogen peroxide and acetic acid, and its chemical formula is C2H4O3. Concentrated liquids are volatile and corrosive. Therefore, they are sold in diluted forms and sometimes have stabilizers added. It is sold as a 35% or 40% solution.
Peracetic acid, also called peroxyacetic acid (PAA), is widely used for its disinfection and sterilization properties.
- Peracetic acid is a powerful oxidizing agent against bacteria, fungi, spores, and viruses. It is highly effective at killing microorganisms, including the coronavirus, and does not allow the development of resistance mechanisms in microbes.
- It is also suitable for removing surface contaminants, especially proteins, from devices.
Peracetic acid is used in the pharmaceutical industry for decontaminating, disinfection, and sterilizing various products, including isolators, vapor-phase producers, and medical instruments (e.g., endoscopes, arthroscopes).
Peroxyacetic was the first germicide utilized for sterilizing isolators. It continues to be used due to its effectiveness, compatibility with plastics, and low cost. Moreover, it is effective at low concentrations; diluted PAA of 50 to 2,000 ppm in solution is used for antimicrobial action. It can work at lower temperatures and in liquid form, making it easy to use.
Given its effectiveness against the COVID-19 virus, its use increased during the pandemic, and demand continues to grow due to the threat of the virus’ reemergence. Consequently, new factories are being set up to meet market demand.
Peracetic acid is considered a sustainable chemical because its decomposition produces only water, oxygen, and biodegradable acetic acid (vinegar). This decomposition process does not generate environmentally harmful substances.
Human Exposure Occurrence
Given its widespread and increasing use, there is potential for both occupational and environmental exposure to peracetic acid.
Peracetic acid, a potent disinfectant, can lead to exposure through inhalation, skin contact, eye contact, and ingestion in occupational settings, especially in facilities where it’s produced or used. Environmental exposure can occur when waste streams release peracetic acid. Safety measures and regulations are essential to mitigate these exposure risks.
Why is Peracetic Acid Hazardous?
Despite its efficacy as a disinfectant and sterilizing agent, peracetic acid can be hazardous for two reasons.
- It is a highly reactive chemical and an explosion risk.
- Peracetic acid exposure can have acute and chronic health effects since it is very corrosive.
Health Effects
Diluted and concentrated peracetic acid can have health effects. The health effects depend on the duration and concentration of the substance, besides other factors.
Acute Effects
The acute effects occur immediately or shortly after exposure:
- Contact with peracetic acid can cause severe eye and skin irritation and even eye damage.
- Inhalation irritates the nose, throat, and respiratory tract, causing coughing and shortness of breath. Concentrated exposure can cause pulmonary edema.
- Very dilute vapor or mist of the peracetic acid can also irritate the eye, throat, nose, and respiratory tract, but symptoms stop once the exposure ceases.
Chronic Effects
Long-term exposure to peracetic acid produces symptoms after some time, can last months to years, and affects the liver and kidneys. It could cause lung cancer in animals.
Drinking and smoking that affect the lungs and kidneys can compound effects caused by exposure to peracetic acid.
Table 1: Safe levels of Peracetic acid exposure to be maintained. (Image credits: https://www.osha.gov/chemicaldata/866)
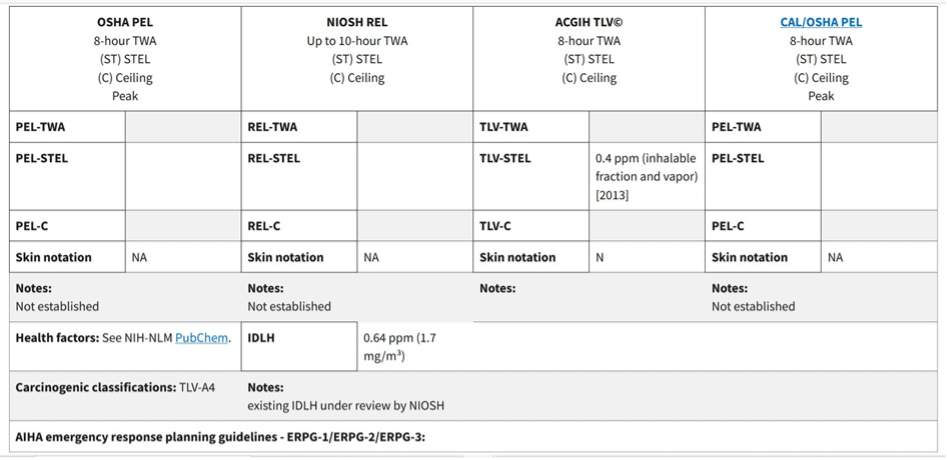
Safe Exposure Limits
Even though peracetic acid can be hazardous, no specific safety standards have been set by the Occupational Safety and Health Administration (OSHA) and the National Institute for Occupational Safety and Health (NIOSH); see Table 1.
The American Conference of Governmental Hygienists (ACGIH) has recommended a “Threshold Limit Value (TLV) of 0.4 ppm (parts per million) as a 15-minute Short-Term Exposure Limit (STEL).” The ACGIH is not legally applicable.
NIOSH is working on determining the PAA concentrations when it is “Immediately Dangerous to Life and Health (IDLH).
Reducing Occupational Exposure
Employers are required to undertake several steps to reduce exposure risk in pharmaceutical factories. The following list indicates some vital steps that can protect workers. For more comprehensive information, check this N.J. information.
- Transportation and storage: Peroxyacetic acid is usually transported and stored in diluted solutions to prevent explosions. The chemical must also be stored in tightly closed containers in cool, well-ventilated areas.
- Labeling: Employers are required to label all peracetic acid containers properly.
- Safety data sheets: Manufacturers and users of peracetic acid should provide comprehensive safety data sheets that outline the potential hazards and safe handling procedures. This information is critical for workers to understand the risks and how to protect themselves.
- Training and information: Employers must also train staff on storing, safe handling, and disposing of peracetic acid. This includes proper emergency response procedures.
- Engineering controls: Facilities should implement engineering controls to reduce exposure, such as local exhaust ventilation systems and containment measures in use and chemical release areas. Adequate ventilation and explosion-proof electrical equipment should be used when handling this chemical.
- Protective gear: People using peracetic acid should wear protective gear like gloves, disposable work clothing, and a full-face respirator with chemical filter cartridges to minimize direct skin contact and inhalation of peracetic acid.
- Monitoring and detection: Routine evaluation of exposure to hazardous substances, including peracetic acid, should be conducted. It could include collecting air samples and using detectors to ensure safe working conditions.
- Occupational exposure monitoring: Workers in facilities where peracetic acid is produced or used should be regularly monitored for exposure. This monitoring can include air sampling to measure airborne peracetic acid concentrations and dermal exposure assessments.
- Environmental release monitoring: Facilities that handle and use peracetic acid should have monitoring systems to detect potential environmental releases. The measures include monitoring effluent discharges and ventilation systems.
- Emergency response: In the event of a spill or release, facilities should have emergency response plans, including using appropriate personal protective equipment and procedures for containing and mitigating the release.
- Regular audits and inspections: Regular audits and inspections of facilities can help identify and correct any deficiencies in exposure control measures.
- Regulatory compliance: Ensure compliance with local, national, and international regulations regarding the handling and disposal of peracetic acid and other hazardous chemicals.
Sensors for Exposure Detection
The risks associated with peracetic acid can be effectively managed by implementing proper monitoring, controls, and safety measures. Fixed and portable sensors that detect trace amounts of the chemical help monitor the air. Interscan’s GasD® 8000 portable gas analyzers offer three options measuring up to 50 ppm with accuracy of 0.01 and 0.1 ppm for people, especially in confined spaces and spills. A safety manager can protect workers and the environment by using enough detection sensors as part of the safety protocols.
Source
CDC. (2016, September 18). Peracetic Acid Sterilization. Retrieved from https://www.cdc.gov/infectioncontrol/guidelines/disinfection/sterilization/peracetic-acid.html
FSIS Environmental, Safety and Health Group. (n.d.) HEALTH HAZARD INFORMATION SHEET Peroxyacetic Acid (PAA). Retrieved from https://www.fsis.usda.gov/sites/default/files/media_file/2020-08/Peroxyacetic-Acid.pdf
Global Peracetic Acid Market Size & forecast [latest]. Markets and Markets. (n.d.). https://www.marketsandmarkets.com/Market-Reports/peracetic-acid-market-1111.html
Peracetic Acid – an overview | ScienceDirect Topics. (n.d.). https://www.sciencedirect.com/topics/pharmacology-toxicology-and-pharmaceutical-science/peracetic-acid
Peracetic acid. Occupational Safety and Health Administration. (n.d.). https://www.osha.gov/chemicaldata/866
N.J. Department of Health and Senior Services. (1998, March). Hazardous Substance Fact Sheet. Retrieved from https://www.nj.gov/health/eoh/rtkweb/documents/fs/1482.pdf


