- Industries must monitor ammonia due to its toxic, corrosive, and flammable properties.
- The ten industries that need to monitor ammonia in the air are ammonia, nitrogenous fertilizers, semiconductor and LED manufacturers, chemical and goods manufacturers, refrigerant system users, livestock farms, wastewater treatment plants, pulp and paper industries, oil refineries, and green energy producers.
- Precision monitoring of ammonia, along with other engineering and administrative controls, is necessary to regulate its levels to keep employees and facilities safe.
Ammonia is a versatile chemical whose applications are increasing, but it is also hazardous. It is among the top three chemicals reported to the EPA (US Environmental Protection Agency) that cause industrial accidents resulting in injuries, deaths, and property damage. EPA notes that 96% of these accidents are easily prevented. Hence, industries that produce or use it should take precautions to avoid exposure and accidents involving ammonia. Industrial hygienists and safety managers can read this article to determine whether their facilities are at risk of ammonia hazards.
Ammonia and Its Applications
Ammonia (NH3) can exist as a solid or a gas. The gas is colorless with a distinct odor. The compound is found naturally and is produced as waste by humans and many animals. It occurs naturally in air, water, and soil through the breakdown of dead plants and animals and manure. In the air and soil, it can occur at concentrations of 1 to 5 parts per billion (ppb), and in rainwater and water bodies at 6ppb. Its levels in nature vary by season and time of day, being highest in summer and spring.
Ammonia is also manufactured artificially in factories. Ammonia is the second-most-produced chemical in the world, and 80% of it is used to produce nitrogenous fertilizers. The rest is used as a feedstock to make nitrogen-containing chemicals and everyday items such as plastics, fabrics, dyes, and pesticides. Novel applications of the compound are increasing and expanding its use. Recently, its application as a carbon-free fuel is also gaining traction.
Why Should Industries Monitor Ammonia
While ammonia is a promising chemical, it is registered by the Occupational Safety and Health Standards (OSHA) under its “List of Highly Hazardous Chemicals, Toxics and Reactives.” Regulatory authorities will therefore investigate any ammonia release.
Around 72% of accidents reported to the EPA between 2004 and 2014 in Iowa, Missouri, Kansas, and Nebraska involved anhydrous ammonia, leading to injuries and deaths of employees, the surrounding community, and emergency personnel. The same EPA report says that 96% of these accidents are preventable through improved procedures and measures, operator training, and better communications of incidents and near-misses.
Minor ammonia releases are common occurrences, and industries need to monitor the gas because it is toxic, corrosive, flammable, and explosive.
Toxicity: Ammonia exposure occurs via inhalation and skin and eye contact. Toxicity effects can occur even at low concentrations during acute exposure. As concentrations and exposure duration increase, the gas’s impact increases. It irritates and burns the eyes and skin. Throat and lung irritation causes coughing, which can progress to pulmonary edema.
Gender, age, lifestyle, and health status can be compounding factors. At 300 to 500 ppm, exposure to ammonia is immediately dangerous to health (IDTH). Exposure to 2500 ppm onwards for over 30 minutes can be fatal. Chronic exposure effects include coughing, phlegm, wheezing, and asthma.
Corrosivity: Ammonia is very corrosive and attacks moist human tissue in the skin, nose, throat, and lungs because, in its anhydrous state, it has a great affinity for water. Ammonia also causes corrosion of metals and alloys like copper and zinc. Therefore, steel and iron are used to store and transport ammonia, but anhydrous ammonia can also attack steel.
Flammability: Ammonia is very flammable at concentrations of 15 to 28% by volume of air. In confined spaces and when transported as compressed gas, where concentrations can reach flammable levels, it can become an explosion hazard when in contact with an ignition source.
Ammonia is identifiable by odor only at concentrations between 5 and 50 ppm. At higher levels, it causes olfactory fatigue or adaptation, making detection challenging. Hence, industries should use precision gas detection through fixed and portable devices as essential engineering controls to keep ammonia levels below permitted levels. Check these New Jersey Health and NIOSH reports for permissible limits and appropriate measures to keep employees safe from ammonia exposure.
The primary industries that need to monitor ammonia in their facilities include ammonia manufacturers, fertilizer producers, refrigeration system users, waste treatment facilities, the paper and pulp industry, chemical and other manufacturing industries, oil refineries, and green marine fuel producers.
-
Ammonia Producers
Ammonia-producing factories rely on complex processes that combine hydrogen and nitrogen at high temperatures and pressures to produce ammonia. Accidents are not uncommon in these conditions. A 2019 study found that human error, followed by valve or piping failure, was the primary cause of ammonia releases. Other causes are component failure, control system failure, noncompliance with operating procedures, inadequate preventive maintenance, lack of training, and harsh operating environments, as shown in Figure 1. OSHA found that even minor releases resulted in burns and deaths.
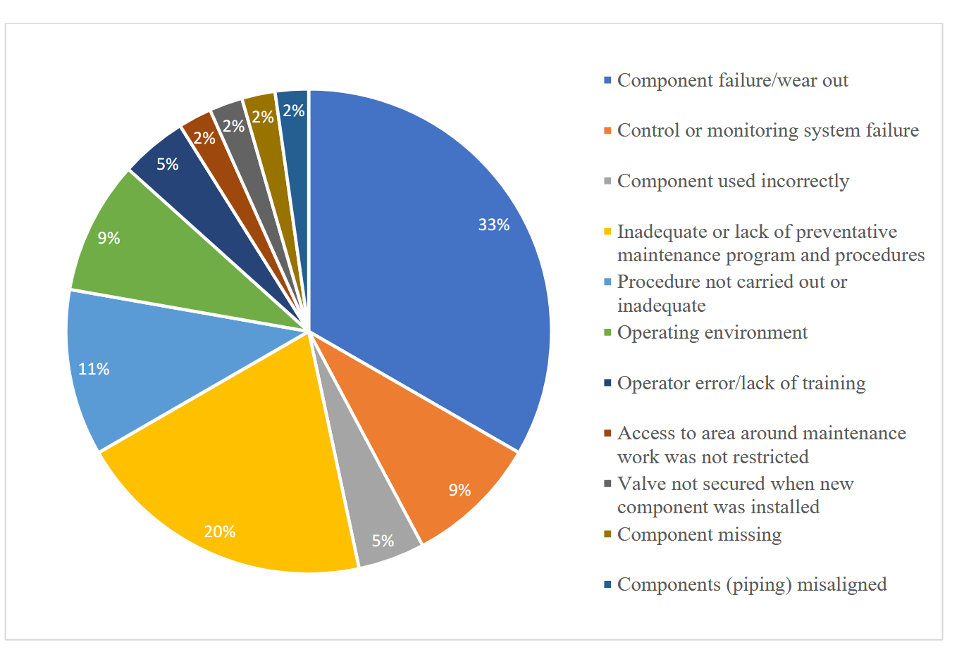
Figure 1: “Causes of ammonia-related accidents,” Jain and Patel 2019. (Image credits: https://engineering.purdue.edu/P2SAC/presentations/documents/Industry-Experience-With-Ammonia-Manufacturing-Plants-Fall-2019.pdf)
-
Nitrogenous Fertilizer Producers
Of the 80% of ammonia used as fertilizer, a third is applied directly to the soil, and the rest is converted into ammonium fertilizers. In fertilizer factories, monitoring ammonia is essential in areas adjacent to ammonia and urea plants, and urea granulation, where active processing occurs. Higher ammonia levels can also be expected near ammonia handling equipment and operational zones. Levels were lowest in the administration and storage spaces, as shown in Figure 2.
In facilities with proper and adequate measures, even the highest concentrations can be kept below the permitted level of 25 ppm. Most modern facilities use various procedures, such as sensors, scrubbers, closed-loop systems, personal protective equipment, automated control systems, biofiltration, ventilation, sealing and insulation, catalytic oxidation, and ammonia absorbers, to control ammonia levels.
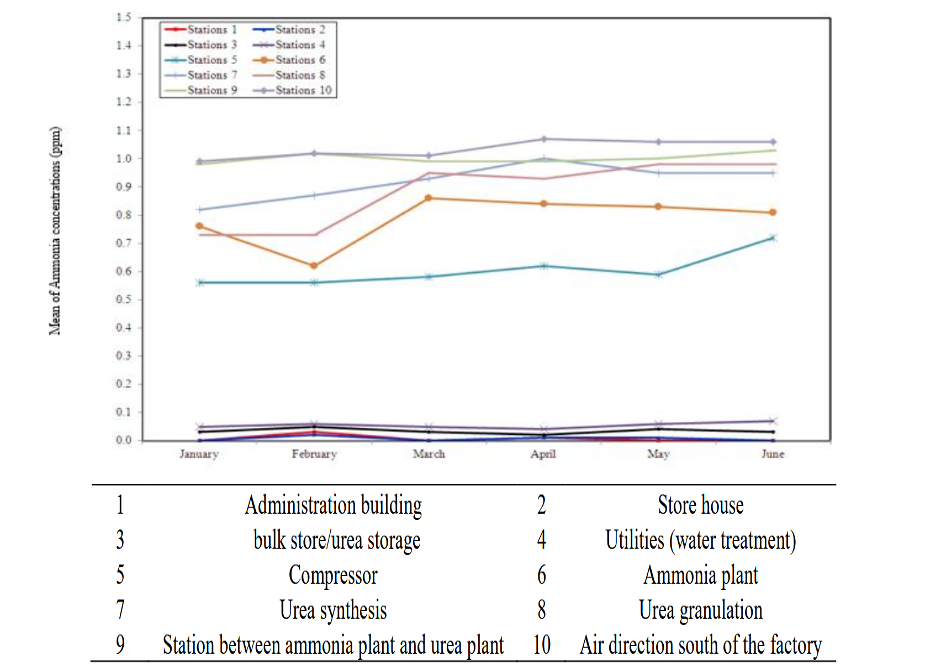
Figure 2. “Ammonia concentrations (ppm) during six months in different stations in modern modified nitrogenous fertilizer factory,” Elhadad et al. 2025. (Credits: 10.21608/asejaiqjsae.2025.438195)
-
Livestock Farms
Ammonia should be monitored in cattle barns and poultry houses because ammonia is produced as a byproduct of animal waste. Livestock management uses nitrogen-rich feed that is not completely metabolized and excreted. So, ammonia is produced during manure decomposition. Ammonia can accumulate in places where animals are fed or kept, and in manure storage facilities. A 2018 study in the USA found that high-density animal farms are hotspots of ammonia emissions, second only to fertilizer producers.
Monitoring is critical in fertilizer production and storage facilities, as well as in barns and poultry houses, to protect animal and worker health from emissions. Proper ventilation can prevent ammonia accumulation and adverse health effects among farm employees. Workers should use personal protective gear (PPE) before entering long-term mature storage pits.
-
Refrigeration
Refrigeration systems use the second-largest amount of ammonia, after fertilizer production. Liquid ammonia is a common refrigerant used in large-scale industrial cooling systems and for air-conditioning in food processing, cold storage, and ice-production facilities.
Table 1: “Ammonia Incident Database; incident causes in the USA and Canada,” Jordan 2020.
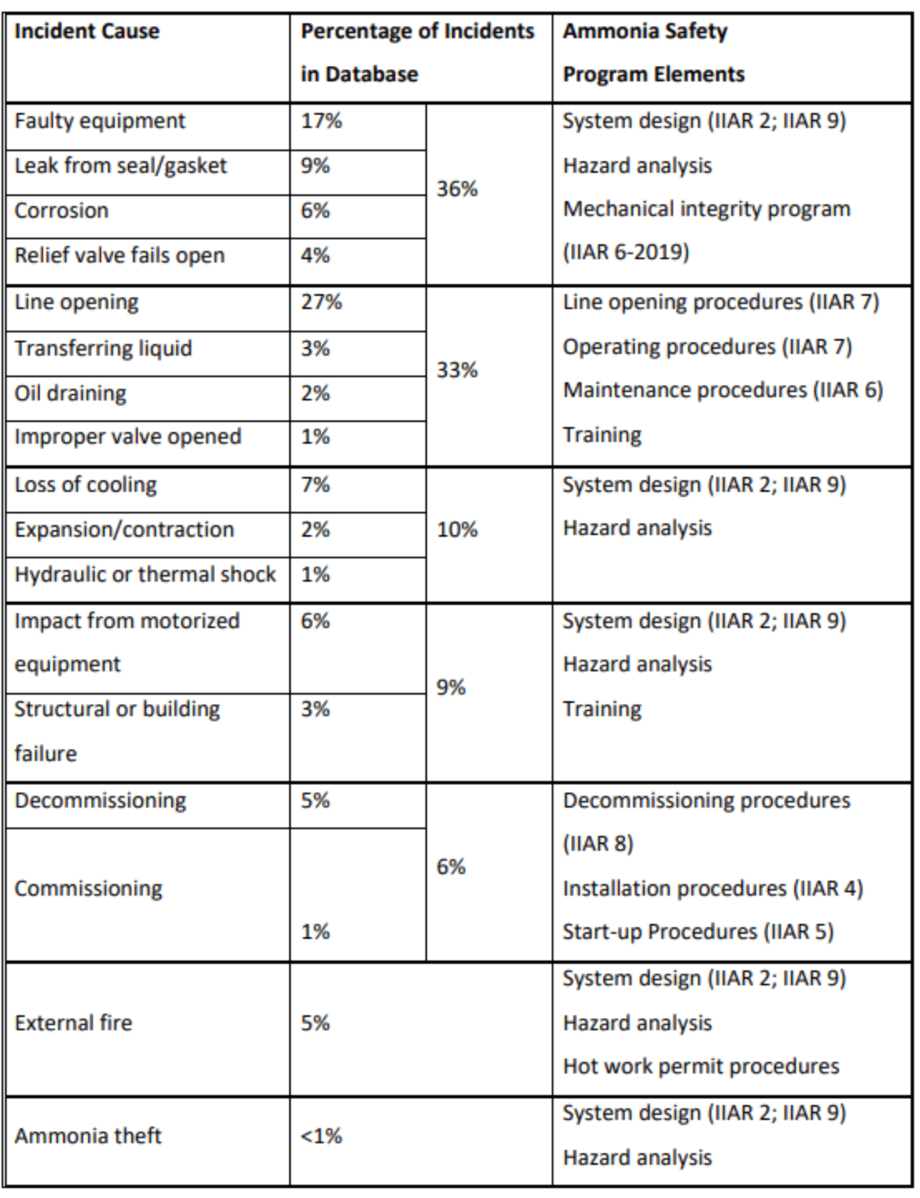
https://iiarcondenser.org/case-history-a-study-of-incidents-in-the-ammonia-refrigeration-industry/
An International Institute of Ammonia Refrigeration (IIAR) report published in 2020 found that half of the incidents involving ammonia occurred outdoors. Indoors, the highest number of ammonia-related accidents occurred in machinery rooms (28%), and 22% in production areas. The main reason was malfunctions in the pressure relief valve. Other equipment failures that can also cause ammonia accidents are leaks from mechanical seals, corrosion, hydraulic and thermal shocks, and safety cutout failures. A detailed list of reasons is provided in Table 1 to help identify potential high-risk areas and ammonia-emission incidents.
-
Wastewater Treatment Plants
Ammonia emissions are produced during sewage and sludge processing in wastewater treatment plants. Hence, monitoring ammonia is essential in these facilities. Other measures that can keep ammonia levels low include using scrubber systems, mitigating cross-contamination among exhaust systems, ventilation, and controlling fumes from storage and process tanks.
-
Petrochemicals Refinery
Ammonia is required for petroleum refining. Ammonia emissions occur in operations such as hydrocracking, catalyst regeneration, and amine treating. It is necessary to control ammonia emissions and leaks during its use, storage, and transport.
Leakage can occur due to valve malfunction or plugging, or defects in pipe flanges, valve stems, and shaft seals. Ammonia’s corrosive action can also cause damage to pipes and tanks due to material failure. It is necessary to ensure that there are no ammonia emissions in areas with high temperatures to prevent fire and explosions.
In addition to monitoring, scrubbing can be used in areas where there is a risk of ammonia emissions.
-
Paper and Pulp Industries
Ammonia is used for pulp bleaching and recovery. It can escape from areas where the processes occur. Several other causes of ammonia accidents have been reported in the pulp and paper industry.
In November 2024, an explosion at the Kuopio fluting mill in Finland was caused by ammonia mixing with hydrogen gas formed in the pulp storage area.
Activities such as welding and grinding above storage tanks, where ammonia accumulation is possible, can lead to explosions. Corrosion of equipment by ammonia in tanks, instrumentation, and control systems has also been reported.
Hence, it is crucial to monitor the air for ammonia, particularly in wet processing sections, and to use scrubbers to control gas levels, thereby protecting workers’ health and preventing damage to facilities.
-
Chemical and Manufacturing
Ammonia is used as an ingredient, agent, or catalyst stabilizer to produce chemicals, synthetic textiles, plastics, rubber, dyes, pharmaceuticals, pesticides, and explosives. It is also used as an alkali to stiffen steel-based materials and for water purification. In all these industries, ammonia sensors will be necessary to monitor the gas for any leakage.
Ammonia leaks can occur due to many causes, such as:
- Equipment: Old equipment and improper maintenance can cause leaks. Corrosion from ammonia, as well as wear and tear, can cause equipment failure of tanks, pipes, and valves.
- Piping systems: The pipes that transport ammonia should be corrosion-resistant; otherwise, they can crack and leak over time.
- Management: Lack of scientific management and failure to adhere to proper operational procedures can result in the unintentional release of ammonia.
- Storage: Ammonia is stored and transported in pressurized cylinders, which must be handled carefully and stored in proper conditions to prevent explosion or leaks.
- Ventilation: Lack of adequate ventilation can lead to ammonia accumulation, which can harm workers, especially if leaks occur. Confined spaces must be entered only after inspection by portable sensors.
-
Green Energy Production
Ammonia can burn as a fuel in standard engines. It can become a non-carbon energy source, helping prevent greenhouse gas emissions and replace fossil fuels. Ammonia can be used directly as a fuel or as a carrier of hydrogen.
Currently, only 1% of energy needs are met by ammonia. However, interest is growing in using it as a fuel for ships and aircraft, as well as for power generation. The use of ammonia as an energy source is expected to increase threefold its 2020 levels by 2050. The production, transport, and storage stages of ammonia will need monitoring to prevent hazards.
-
Semiconductor and LED Industries
Manufacturing in the semiconductor industry requires strict control of air purity, as several chemicals, including 30 specialty gases, are used for film deposition, etching, cleaning, and as dopants and inert carriers.
Aqueous ammonia, also known as ammonia hydroxide, is used for precision cleaning in electronics production, including semiconductors and LEDs. However, it has to be handled carefully due to its corrosivity and volatility. It is usually handled in closed systems using PPE and air monitoring.
Semiconductors could also use ammonia to react with silane and produce silicon nitride for film deposition. While LEDs use ammonia to form gallium nitride, to convert electricity into light.
Even trace amounts of remnant ammonia can damage sensitive equipment through corrosion, and airborne ammonia can affect processes such as etching and doping. So, precision monitoring of ammonia and scrubbing are essential to keep ammonia levels low and safeguard production and equipment integrity.
Try Interscan
Interscan offers AccuSafe and GASD 8000, fixed and portable ammonia gas analyzers, respectively. Both systems have precision gas sensors that can measure ppb concentration of the gas, making them suitable for all industries, even the electronics sector. The fixed system has ten sensor modules connected to a central controller via Modbus TCP/P for continuous monitoring, data logging, transmissions, and remote monitoring. The unit can be integrated with automated control systems. The portable gas sensor is small yet precise enough for use in confined spaces and for detecting ammonia in high-risk areas.
Contact us at Interscan to learn more about our ammonia sensors for safety applications.
Sources
ACS. (2021, Feb 8). Molecule of the Week Archive Ammonia. Retrieved from https://www.acs.org/molecule-of-the-week/archive/a/ammonia.html
ATSDR. (n.d.). Public Health Statement for Ammonia. Retrieved from https://wwwn.cdc.gov/TSP/PHS/PHS.aspx?phsid=9&toxid=2
Amhamed, A. I., Shuibul Qarnain, S., Hewlett, S., Sodiq, A., Abdellatif, Y., Isaifan, R. J., & Alrebei, O. F. (2022). Ammonia Production Plants—A Review. Fuels, 3(3), 408-435. https://doi.org/10.3390/fuels3030026
Elhadad, S., Mansour, T., Elshibiny, I., & Elmarakby, F. (2025). Limitation of Ammonia Exposure Among Workers in Fertilizers Industry using Modern Modified Technology Towards Sustainability. Alexandria Science Exchange Journal, 46(2), 507-519. doi: 10.21608/asejaiqjsae.2025.438195
Jordan, P.R. (2020, August). Case History: A Study of Incidents in the Ammonia Refrigeration Industry. Retrieved from https://iiarcondenser.org/case-history-a-study-of-incidents-in-the-ammonia-refrigeration-industry/
Kang, S., Kim, G., Roh, J., & Jeon, E. C. (2022). Ammonia Emissions from NPK Fertilizer Production Plants: Emission Characteristics and Emission Factor Estimation. International journal of environmental research and public health, 19(11), 6703. https://doi.org/10.3390/ijerph19116703
Mallouppas, G., Ioannou, C., & Yfantis, E. A. (2022). A review of the latest trends in the use of green ammonia as an energy carrier in maritime industry. Energies, 15(4), 1453.
UK Health Security Agency. (2024, Oct 23). Guidance Ammonia: toxicological overview. Retrieved from https://www.gov.uk/government/publications/ammonia-properties-incident-management-and-toxicology/ammonia-toxicological-overview
Van Damme, M., Clarisse, L., Whitburn, S., Hadji-Lazaro, J., Hurtmans, D., Clerbaux, C., & Coheur, P. F. (2018). Industrial and agricultural ammonia point sources exposed. Nature, 564(7734), 99-103.
Smith, G. (2022, July 17). Ammonia storage hazards and uses. Retrieved from https://insights.globalspec.com/article/18908/ammonia-storage-hazards-and-uses
Agency for Toxic Substances and Disease Registry (US). (2004). Toxicological Profile for Ammonia. Atlanta (GA): POTENTIAL FOR HUMAN EXPOSURE. Available from: https://www.ncbi.nlm.nih.gov/books/NBK598711/
Yüzbaşıoğlu, A. E., Avşar, C., & Gezerman, A. O. (2022). The current situation in the use of ammonia as a sustainable energy source and its industrial potential. Current Research in Green and Sustainable Chemistry, 5, 100307.


