- Artificial intelligence (AI) and machine learning integration in sensors are used to increase accuracy and pattern detection to make predictions of gas concentrations.
- Smart, smaller, and wearable wireless gas sensor innovations are increasing.
- Nanomaterials with unique morphologies and gas-sensing abilities are also being tested and analyzed for broad applications.
In many industries, gas sensors are essential for environmental monitoring to maintain occupational safety and health. Gas detection is rising, and the global market for sensors was estimated at 3.3 billion USD in 2024 and is expected to grow at a CAGR of 4.3% in the next decade. Technology is also changing to match regulations that are getting more stringent to protect workers, local communities, and the environment. This article discusses five innovations and trends in 2024 that can make hazardous gas monitoring more accurate and reliable.
-
Multi-Layer Perceptron Neural Network for Mine Gas Monitoring Data Analysis
Though demand is declining, coal remains a significant source of energy. Mining operations seek to maintain high safety standards and must deal with one of the foremost safety challenges- the high methane concentrations in coal deposits. Methane is highly flammable and explosive, and mine operators want to prevent its accumulation; however, concentrations above permissible levels can still occur due to the nature of mining. It leads to automatic power disconnections that lead to work interruptions. The ability to forecast methane concentrations to support ventilation systems and other operational parameters can increase mining safety, efficiency, and continuity.
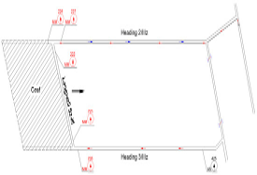
1a) Location of sensors
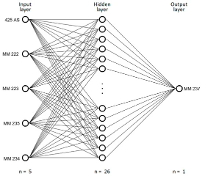
1b) MLP neural network based on sensor data
Figure 1: “a) Schematic of the ventilation of the longwall under study with the location of the sensors. b) Structure and parameters of the MLP 5-26-1 neural network model for predicting methane concentration at the longwall outlet,” Tutak et al. 2024. (Image credits: after )
Research findings
Tutak et al. 2024 attempted to apply artificial neural networks. This machine learning technique has the advantage of being able to analyze vast amounts of time series data without information on the correlation between variables. The approach eliminates costly and complex models. However, few neural network applications exist for coal mining.
Tutak et al. 2024 developed a machine-learning model based on continuous ventilation parameters in a given area, capable of forecasting short-term methane concentrations after 5, 10, 15, 30, and 60 minutes to predict emergencies.
Neural Network selection, training, and testing were done using only longwall data from the mine’s automatic gas monitoring system, namely from MM 222, 223, 234, 235, and As 425 sensors. No mining or geological parameters were used. The artificial neural network models used were Multi-Layer Perceptron (MLP). The goal was to analyze methane concentrations at MM 237 at the longwall outlet, see Figure 1a and Ib.
The model’s effectiveness in forecasting depends on input data and timing. The most accurate predictions were obtained for 5-minute forecasts. While predictions after 10, 15, 30, and 60 minutes were less precise, they were acceptable since the “Mean Absolute Percentage Error” was less than 18%. The model adapted to the dynamic and rapid changes in data.
Takeaway: The MLP models are simple and versatile for practical implementation to keep methane below permissible levels and improve mining safety. Artificial neural networks are generally helpful for their predictive maintenance capability, which can reduce industry downtime.
-
Smart Gas Sensor Technology
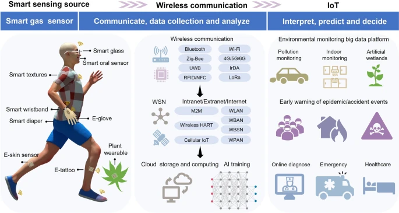
Figure 2: “Full-spectrum operation procedures of smart gas sensors. Adapted illustration 81383831,” Zong et al. (2024). (Image credits: https://link.springer.com/article/10.1007/s40820-024-01543-w)
In the past decades, gas sensors have become essential for indoor and atmospheric air monitoring in personal, professional, and industrial settings. Two broad types, electrically transduced (electronic) and optically transduced sensors, are used for this purpose. Applications of electrically transduced sensors are growing due to compatibility with wireless communication and electronics, rapid and real-time analysis and monitoring, and portability.
Review
Zong et al. (2024) review the progress made in the last decade in smart portable and wearable electronic and optoelectronic sensors for gas monitoring.
- Electronic sensors: These sensors use physical and electronic changes as signals and include electrochemical sensors, capacitors, field-effect transistors, and chemiresistors. These are flexible sensors and have ppb-level detection. However, cross-sensitivity between chemicals with similar chemical structure is a challenge.
- Optoelectronic sensors: Differences in color response or visual fluorescence due to variations in a target gas’ optical property are used as the signal.
The improvements in selectivity, sensitivity, and accuracy in these two gas sensors are due to machine-learning algorithms, innovative engineering, and sensor array construction, which have produced high-performance smart gas monitoring.
Machine-learning (ML) algorithms enable high prediction accuracy even for unknown gases. ML aids gas sensors in learning from their environments and adapting to dynamic conditions, providing more reliable readings. Gas sensors’ integration into an Internet of Things (IoT), such as Bluetooth, Z-Wave, WiFi, etc., allows further data processing for detection, prediction, and applications in early warning systems for remote gas monitoring, see Figure 2.
Takeaway: The state-of-the-art sensor applications have multiplied as they integrate with smart terminals and integrated technology, allowing for wireless, smart gas sensing with flexible and wearable gas sensors.
-
Silver Nanowire-Decorated Silicon Nanomembrane Wireless Wearable Sensor System
The research by Shin et al. (2024) provides an example of one of the new flexible electronics for industry and academia. One of the vital roles of these wearable sensors is the ability for continuous environmental monitoring of hazardous gases in industries and healthcare. Ammonia (NH3) is a common hazardous gas found in industry and daily life, and strict and stringent safety standards regulate it.
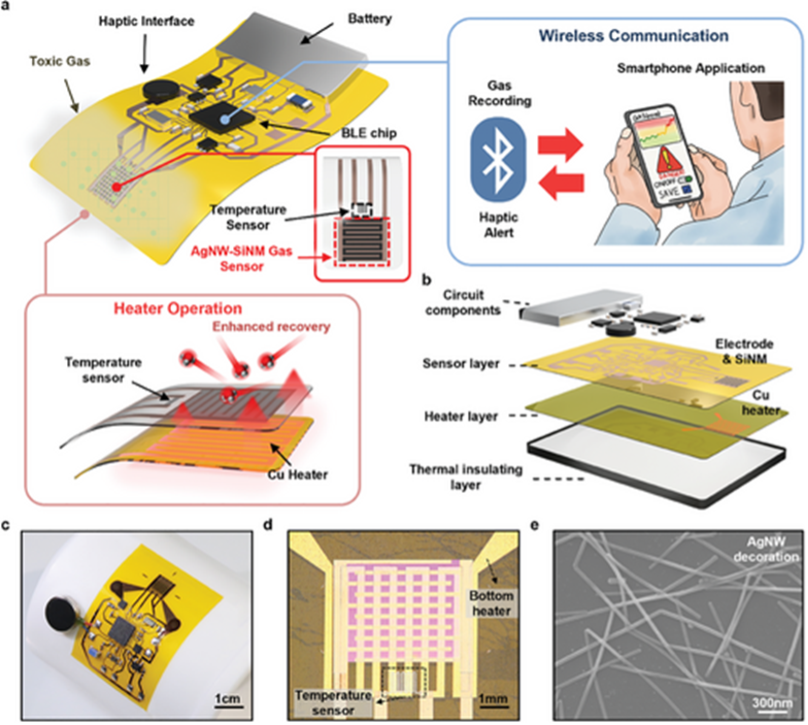
Figure 3: “A fully integrated wearable wireless gas sensing system for real-time monitoring of ambient toxic gases. a) Overview of the wearable gas sensing system incorporating a silver nanowire-decorated silicon nanomembrane (AgNW-SiNM) sensor, an ultrathin flexible heater, and a wireless communication system. b) Exploded view of the device with circuit components, AgNW-SiNM sensor layer, Cu heater layer, and thermal insulating layer. c) A photograph of the fully integrated device attached to the curved surface of a beaker with a 20 mm bending radius. d) A magnified view of the vertically stacked flexible heater and SiNM sensors. e) SEM image of silver nanowires decorated on silicon nanomembrane,” Shin et al. (2024). https://doi.org/10.1002/adfm.202419110
Research Findings
Several approaches, technologies, and materials are used to make wearable sensors. In this study, Shin et al. (2024) developed a chemiresistive gas sensor made of “silver nanowires-decorated silicon nanomembrane (AgNW-SiNM”), which had a high selectivity to ammonia. The scientists combined the sensor with a flexible Joule heater to improve the sensor’s performance and a wireless circuit for Bluetooth communication, see Figure 3.
The heater operated stably in a range from ambient temperature to 120°C. So, the target temperature of 85°C is quickly reached, and the PDMS heat insulating layer protects the wearer from the warmth. The high temperature enhances adsorption/desorption rates, improving the sensor’s recovery time to NH3 exposure.
An amount of 5 wt% AgNW-SiNM was identified as optimum decoration through experiments and modeling. The gas sensor gave stable responses of ≈1.19, 1.47, and 1.83 to ammonia exposure of 1, 5, and 10 ppm, respectively. Mechanical deformation did not affect performance, showing it was suitable as a wearable sensor. Real-time Bluetooth communication supported data transmission to portable electronics such as Android applications. The haptic interface provided early warnings of ammonia levels in the atmosphere for timely evacuations.
Takeaway: The new wearable wireless gas sensing system can provide an early warning system for toxic gas monitoring and help diagnose respiratory problems accurately.
-
Overcoming Cross-Sensitivity: Pattern Recognition Methods
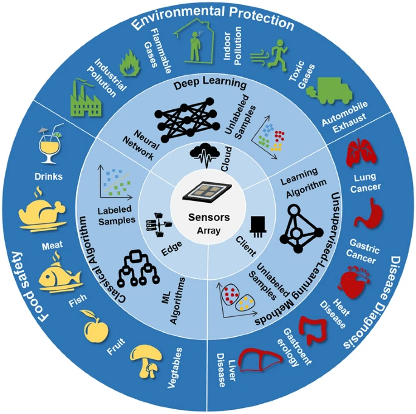
Figure 4: The application of various models in multiple sectors, as summarized by Mei et al. (2024). (Image credits: https://link.springer.com/article/10.1007/s40820-024-01489-z)
Chemiresistive sensors are widely used for hazardous chemical detection as they are helpful for several gases, are easy to manufacture, and are cost-effective.
These sensors operate in environments that have many gases. The accuracy of chemiresistive sensors depends on their sensing mechanism, which also causes cross-sensitivity because they react with other gases. Chemiresistive sensors use the oxygen adsorption model to sense gases. However, any reactive gas can be oxidized or reduced, changing the resistance or conductivity of the sensor and leading to cross-sensitivity of metal oxide semiconductor (MOS) sensors to many gases and VOCs. The cross-sensitivity of these sensors limits their use in gas monitoring as part of an artificial olfactory system, which uses multiple sensors.
Review
Mei et al. (2024) review various pattern recognition techniques to overcome chemiresistive gas sensors’ cross-sensitivity, see Figure 4. The choice of the pattern recognition method for data analysis to reduce errors and improve reliability becomes crucial, so the scientists have summarized the properties and suitability of the models.
Machine learning (ML): These algorithms are suitable for scenarios with resource and data limitations for chemical monitoring. Its advantages are low computational complexity and strong interpretability.
Artificial neural networks: Models such as CNN, FVV, and RNN are excellent for cases with extensive data sets involving multiple gases. These algorithms can analyze data with intricate relationships and identify patterns by extracting features.
Ensemble Learning: Ensemble learning methods consist of multiple machine learning models with better predictive performance and accuracy. These models are best suited for high data interference and complexity through model fusion.
The scientists suggest testing several model types to find the best strategy. They found that the most recent advances on this topic pertained to gas detection for environmental monitoring, food safety, and medical diagnosis.
Takeway: The study has summarized the suitability of various algorithms and offers insights for appropriate model selection in overcoming sensor cross-sensitivity for gas recognition applications.
-
Electrospun 1D Nanostructures for Chemoresistive Gas Sensors
Chemoresistive gas sensors can differ based on the sensing material, method, and application area. Depending on the materials used, these sensors can be semiconductor metal oxide, carbon, conductive polymers, and composites. Carbon-based materials, including nanotubes, reduced graphene oxide, and graphene, are increasingly used for their unique mechanical and electrical properties.
The standard methods used are electrolysis, pyrolysis, chemical deposition, electrospinning, etc. Electrospinning is the most effective and versatile method. The nanofibers it produces can be mats or arrays, and the fibers can have diameters from nanometers to micrometers.
Review
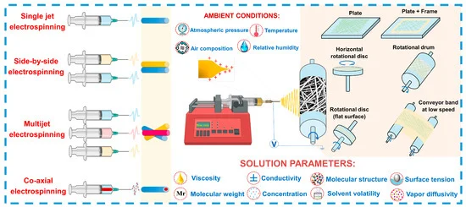
Figure 5: “Schematic illustration of the electrospinning process,” Imash et al. (2024). (Image credits: https://www.mdpi.com/1424-8220/24/21/6797)
Imash et al. (2024) have reviewed the recent advances in 1D nanomaterials, such as nanofibers and nanowires produced from electrospinning. The fine dimensions of 1-D nanomaterials allow precise control over the material morphology for specific applications.
Electrospinning can produce nanofibers and nanowires with unique morphologies that produce high surface area and porous structures, which enhance gas sensing properties like selectivity, sensitivity, response time, and stability.
Nanofiber-based sensors’ selectivity for specific gases makes large-scale production difficult. One explored solution is improving nanofiber morphology, combining it with other materials and active elements. The nanofiber-based composites achieve high selectivity and sensitivity by leveraging the properties of various materials, allowing the detection of many gases.
Testing and analyzing new materials and production methods will be necessary to overcome their limitations and broaden their applications.
Takeaway: Focusing on electrospun nanofiber morphology and their impact on gas sensing properties will be vital to include them in gas monitoring technologies.
Industry Standard Gas Analyzers
Several gas analyzers that allow the detection and monitoring of hazardous gases in trace amounts to keep their levels under permissible limits already exist on the market. Interscan’s fixed and portable gas detectors, with sensors for over 20 gases, can monitor gases at ppb levels when required. They can be used together in the Accusafe fixed systems to allow multiple gas detection simultaneously. Interscan gas sensors are industry standards trusted and used in several industries.
Contact us for more information on Interscan’s portable and fixed gas systems.
Sources
Imash, A., Smagulova, G., Kaidar, B., Keneshbekova, A., Kazhdanbekov, R., Velasco, L. F., & Mansurov, Z. (2024). Chemoresistive Gas Sensors Based on Electrospun 1D Nanostructures: Synergizing Morphology and Performance Optimization. Sensors, 24(21), 6797.
Mei, H., Peng, J., Wang, T., Zhou, T., Zhao, H., Zhang, T., & Yang, Z. (2024). Overcoming the limits of cross-sensitivity: pattern recognition methods for chemiresistive gas sensor array. Nano-micro letters, 16(1), 269.
Shin, J., Kim, K., Min, I. S., Sang, M., Lee, J. Y., Hwang, K., … & Yu, K. J. (2024). A Wireless Wearable Sensor System Based on a Silver Nanowire‐Decorated Silicon Nanomembrane for Precise and Continuous Hazardous Gas Monitoring. Advanced Functional Materials, 2419110.
Tutak, M., Krenicky, T., Pirník, R., Brodny, J., & Grebski, W.W. (2024). Predicting Methane Concentrations in Underground Coal Mining Using a Multi-Layer Perceptron Neural Network Based on Mine Gas Monitoring Data. Sustainability,16, 8388. https://doi.org/10.3390/su16198388
Zong, B., Wu, S., Yang, Y., Li, Q., Tao, T., & Mao, S. (2025). Smart gas sensors: recent developments and future prospective. Nano-Micro Letters, 17(1), 54.

